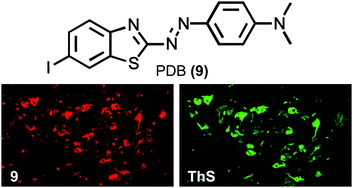
Chronic obstructive pulmonary disease (COPD) is caused by emphysema and chronic bronchitis that damages the airways of the lungs leading to significantly reduced air flow and is predicted to be the third largest cause of death by 2030. Symptomatic relief is given by prescribing up to three different drugs for concurrent use, but the complexity of combining three different drugs that operate via three distinct mechanisms into a single device for inhalation dosing, such that patient compliance is high, is considerable.
Lyn Jones et al. from Pfizer have sought to facilitate the triple therapy concept by pursuing a strategy to incorporate muscarinic antagonism and β2 agonism into a single molecule, such that combination with an inhaled corticosteroid could be achieved in a single dry powder inhaler device.
Compound 15 combined high metabolic clearance, low synthetic complexity, low oral bioavailability, desirable material properties and an impressive therapeutic index over haemodynamic effects, and these characteristics led to its nomination as a clinical candidate (PF-4348235).
This molecule has great potential to ease the medicines burden for COPD sufferers, so read about it now in MedChemComm.
This HOT article is free to access.
Optimized glucuronidation of dual pharmacology β-2 agonists/M3 antagonists for the treatment of COPD
Laura Hilton, Rachel Osborne, Amy S. Kenyon, Helen Baldock, Mark E. Bunnage, Jane Burrows, Nick Clarke, Michele Coghlan, David Entwistle, David Fairman, Neil Feeder, Kim James, Rhys M. Jones, Nadia Laouar, Graham Lunn, Stuart Marshall, Sandra D. Newman, Sheena Patel, Matthew D. Selby, Fiona Spence, Emilio F. Stuart, Susan Summerhill, Michael A. Trevethick, Karen N. Wright, Michael Yeadon, David A. Price and Lyn H. Jones
Med. Chem. Commun., 2011, Advance Article
DOI: 10.1039/C1MD00140J












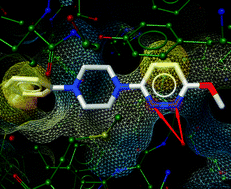
 In this HOT paper David S. Millan and colleagues at Pfizer R&D explore the influence of intramolecular hydrogen bonding on the membrane permeability and bioavailability of some of these drugs. By determining the propensity of molecules to form intramolecular hydrogen bonds they are able to revise previous assumptions about where certain molecules sit in chemical space. They find that when hydrogen bonding is taken into account these drugs sit much closer to rule of five space – thereby explaining the successful absorption of drugs that otherwise violate rule of five principles.
In this HOT paper David S. Millan and colleagues at Pfizer R&D explore the influence of intramolecular hydrogen bonding on the membrane permeability and bioavailability of some of these drugs. By determining the propensity of molecules to form intramolecular hydrogen bonds they are able to revise previous assumptions about where certain molecules sit in chemical space. They find that when hydrogen bonding is taken into account these drugs sit much closer to rule of five space – thereby explaining the successful absorption of drugs that otherwise violate rule of five principles.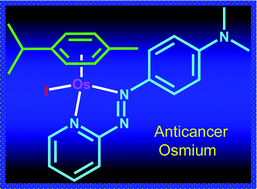 The majority of therapeutics clinically available for cancer treatment are platinum-based and work via a DNA alkylation mechanism that leads to cell apoptosis. But a number of cancers are resistant to apoptosis and not treatable with platinum-based drugs.
The majority of therapeutics clinically available for cancer treatment are platinum-based and work via a DNA alkylation mechanism that leads to cell apoptosis. But a number of cancers are resistant to apoptosis and not treatable with platinum-based drugs.