Howard C. Hang (The Rockefeller University) et al.‘s review article reflects the latest updates on Type III secretion systems (T3SSs) inhibitors. ‘T3SSs are central to the virulence of many human Gram-negative pathogens such as Salmonella, Shigella, Pseudomonas, enteropathogenic Escherichia coli (EPEC), enterohemorrhagic E. coli,(EHEC), Vibrio, Yersinia, and Chlamydia’ explains Hang, and are exciting targets for anti-bacterial development.
![GA[3]](https://blogs.rsc.org/md/files/2012/11/GA3.gif)
The article not only provides the reader with a comprehensive view of the severall classes of small molecules that inhibit the secretion and translocation of bacterial effector proteins, their mode of action and prospects for clinical development, but also brings insights into the different methods developed to allow the screening of very large libraries of molecules.
A must-read for anyone interested in bacterial virulence and small molecule inhibitors.
Small molecules aimed at type III secretion systems to inhibit bacterial virulence
Lun K. Tsou, Paul D. Dossa and Howard C. Hang
Med. Chem. Commun., 2012, DOI: 10.1039/C2MD20213A
|
This review is part of MedChemComm’s New Talent themed issue: Highlighting medicinal chemistry research in its broadest sense and showcasing the strength of research being carried out by tomorrow’s leaders in the field: view the collection grow |











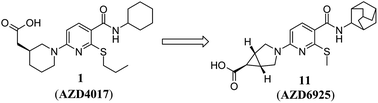
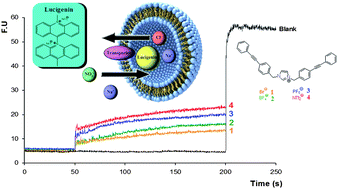
 On the cover of this month’s issue is an article from our
On the cover of this month’s issue is an article from our 
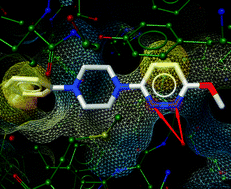 Expanding the horizon of chemotherapeutic targets: From MDM2 to MDMX (MDM4)
Expanding the horizon of chemotherapeutic targets: From MDM2 to MDMX (MDM4)
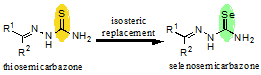
![GA[3]](https://blogs.rsc.org/md/files/2011/11/GA31.gif)