The articles below are some of the most read MedChemComm articles in April, May and June 2017.
Review Articles:
Multivalent glycoconjugates as vaccines and potential drug candidates
Sumati Bhatia, Mathias Dimde and Rainer Haag
DOI: 10.1039/C4MD00143E
Surface plasmon resonance for the characterization of bacterial polysaccharide antigens: a review
Barbara Brogioni and Francesco Berti
DOI: 10.1039/C4MD00088A
Recent advances in the rational design and optimization of antibacterial agents
Jesse A. Jones, Kristopher G. Virga, Giuseppe Gumina and Kirk E. Hevener
DOI: 10.1039/C6MD00232C
Polypharmacology modelling using proteochemometrics (PCM): recent methodological developments, applications to target families, and future prospects
Isidro Cortés-Ciriano, Qurrat Ul Ain, Vigneshwari Subramanian, Eelke B. Lenselink, Oscar Méndez-Lucio, Adriaan P. IJzerman, Gerd Wohlfahrt, Peteris Prusis, Thérèse E. Malliavin, Gerard J. P. van Westen and Andreas Bender
DOI: 10.1039/C4MD00216D
Protein–ligand (un)binding kinetics as a new paradigm for drug discovery at the crossroad between experiments and modelling
M. Bernetti, A. Cavalli and L. Mollica
DOI: 10.1039/C6MD00581K
Structural biology and chemistry of protein arginine methyltransferases
Matthieu Schapira and Renato Ferreira de Freitas
DOI: 10.1039/C4MD00269E
Small molecule-mediated protein knockdown as a new approach to drug discovery
Christopher P. Tinworth, Hannah Lithgow and Ian Churcher
DOI: 10.1039/C6MD00347H
Chemical probes and inhibitors of bromodomains outside the BET family
Moses Moustakim, Peter G. K. Clark, Duncan A. Hay, Darren J. Dixon and Paul E. Brennan
DOI: 10.1039/C6MD00373G
Amphiphilic designer nano-carriers for controlled release: from drug delivery to diagnostics
Malinda Salim, Hiroyuki Minamikawa, Akihiko Sugimura and Rauzah Hashim
DOI: 10.1039/C4MD00085D
2-[18F]Fluoroethyl tosylate – a versatile tool for building 18F-based radiotracers for positron emission tomography
Torsten Kniess, Markus Laube, Peter Brust and Jörg Steinbach
DOI: 10.1039/C5MD00303B
Structural features of many circular and leaderless bacteriocins are similar to those in saposins and saposin-like peptides
K. M. Towle and J. C. Vederas
DOI: 10.1039/C6MD00607H
Bacterial lipid membranes as promising targets to fight antimicrobial resistance, molecular foundations and illustration through the renewal of aminoglycoside antibiotics and emergence of amphiphilic aminoglycosides
Marie-Paule Mingeot-Leclercq and Jean-Luc Décout
DOI: 10.1039/C5MD00503E
Research Articles:
Towards understanding cell penetration by stapled peptides
Qian Chu, Raymond E. Moellering, Gerard J. Hilinski, Young-Woo Kim, Tom N. Grossmann, Johannes T.-H. Yeh and Gregory L. Verdine
DOI: 10.1039/C4MD00131A
Design and synthesis of DNA-encoded libraries based on a benzodiazepine and a pyrazolopyrimidine scaffold
M. Klika Škopić, O. Bugain, K. Jung, S. Onstein, S. Brandherm, T. Kalliokoski and A. Brunschweiger
DOI: 10.1039/C6MD00243A
Methods of protein surface PEGylation under structure preservation for the emulsion-based formation of stable nanoparticles
Lydia Radi, Matthias Fach, Mirko Montigny, Elena Berger-Nicoletti, Wolfgang Tremel and Peter R. Wich
DOI: 10.1039/C5MD00475F
Identification of imidazo[1,2-b]pyridazine TYK2 pseudokinase ligands as potent and selective allosteric inhibitors of TYK2 signalling
R. Moslin, D. Gardner, J. Santella, Y. Zhang, J. V. Duncia, C. Liu, J. Lin, J. S. Tokarski, J. Strnad, D. Pedicord, J. Chen, Y. Blat, A. Zupa-Fernandez, L. Cheng, H. Sun, C. Chaudhry, C. Huang, C. D’Arienzo, J. S. Sack, J. K. Muckelbauer, C. Chang, J. Tredup, D. Xie, N. Aranibar, J. R. Burke, P. H. Carter and D. S. Weinstein
DOI: 10.1039/C6MD00560H
Exploring the links between peptoid antibacterial activity and toxicity
H. L. Bolt, G. A. Eggimann, C. A. B. Jahoda, R. N. Zuckermann, G. J. Sharples and S. L. Cobb
DOI: 10.1039/C6MD00648E
Nonomuraea sp. ATCC 55076 harbours the largest actinomycete chromosome to date and the kistamicin biosynthetic gene cluster
Behnam Nazari, Clarissa C. Forneris, Marcus I. Gibson, Kyuho Moon, Kelsey R. Schramma and Mohammad R. Seyedsayamdost
DOI: 10.1039/C6MD00637J
Structure-based virtual screening for fragment-like ligands of the G protein-coupled histamine H4 receptor
Enade P. Istyastono, Albert J. Kooistra, Henry F. Vischer, Martien Kuijer, Luc Roumen, Saskia Nijmeijer, Rogier A. Smits, Iwan J. P. de Esch, Rob Leurs and Chris de Graaf
DOI: 10.1039/C5MD00022J
Human lysosomal acid lipase inhibitor lalistat impairs Mycobacterium tuberculosis growth by targeting bacterial hydrolases
J. Lehmann, J. Vomacka, K. Esser, M. Nodwell, K. Kolbe, P. Rämer, U. Protzer, N. Reiling and S. A. Sieber
DOI: 10.1039/C6MD00231E
Design and synthesis of potent and selective inhibitors of BRD7 and BRD9 bromodomains
Duncan A. Hay, Catherine M. Rogers, Oleg Fedorov, Cynthia Tallant, Sarah Martin, Octovia P. Monteiro, Susanne Müller, Stefan Knapp, Christopher J. Schofield and Paul E. Brennan
DOI: 10.1039/C5MD00152H
A fundamental view of enthalpy–entropy compensation
Ulf Ryde
DOI: 10.1039/C4MD00057A
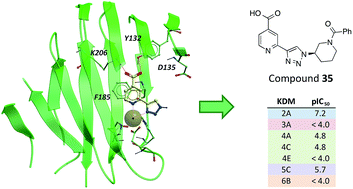
Optimisation of a triazolopyridine based histone demethylase inhibitor yields a potent and selective KDM2A (FBXL11) inhibitor
Katherine S. England, Anthony Tumber, Tobias Krojer, Giuseppe Scozzafava, Stanley S. Ng, Michelle Daniel, Aleksandra Szykowska, KaHing Che, Frank von Delft, Nicola A. Burgess-Brown, Akane Kawamura, Christopher J. Schofield and Paul E. Brennan
DOI: 10.1039/C4MD00291A
From linked open data to molecular interaction: studying selectivity trends for ligands of the human serotonin and dopamine transporter
Barbara Zdrazil, Eva Hellsberg, Michael Viereck and Gerhard F. Ecker
DOI: 10.1039/C6MD00207B
Keep up-to-date with the latest issues of MedChemComm with our E-alerts












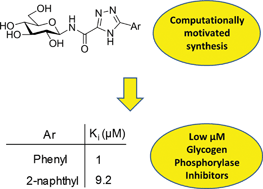
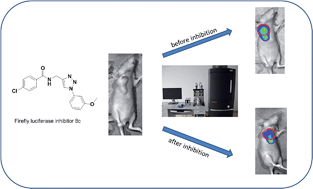
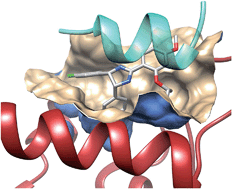
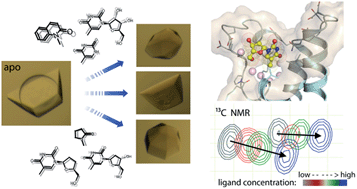

![GA[8]](https://blogs.rsc.org/md/files/2012/11/GA8.gif)