
Broccoli and Brussels sprouts contain compounds that can inhibit the growth of bacteria that cause disease
Chemists from Israel say that the isothiocyanates sulforaphane and erucin, found in brassicaceae vegetables such as broccoli, Brussels sprouts, cabbage and cauliflower, inhibit growth of the disease-causing bacteria Pseudomonas aeruginosa.
Bacterial quorum sensing (QS) is the method by which bacteria communicate. Instead of language, they release signalling molecules into the environment and a single cell can sense the number of other local bacteria. By using QS, bacteria can coordinate their behaviour through gene expression and adapt to changing environmental conditions. With bacteria such as MRSA and Pseudomonas aeruginosa developing antibiotic resistance, alternative strategies to inhibit bacterial growth are necessary and QS inhibition has been suggested as a new strategy to prevent bacterial growth.
The team, led by Michael Meijler from Ben-Gurion University of the Negev, Be’er-Sheva, found that isothiocyanates from broccoli inhibit quorum sensing. ‘This might signal to the bacteria that conditions for colonisation are not optimal,’ he explains. ‘The benefits of eating vegetables to human health in general have been known for quite some time now. At the molecular level, not a great deal is known yet, providing a fertile ground for fundamental research.’
Read the entire Chemistry World story
And then read the full MedChemComm paper:
Sulforaphane and erucin, natural isothiocyanates from broccoli, inhibit bacterial quorum sensing
Hadas Ganin, Josep Rayo, Neri Amara, Niva Levy, Pnina Krief and Michael M. Meijler
DOI: 10.1039/C2MD20196H















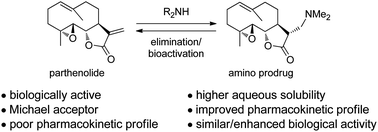
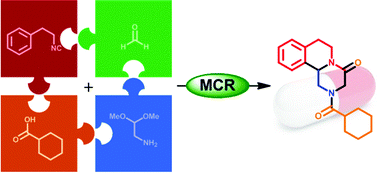
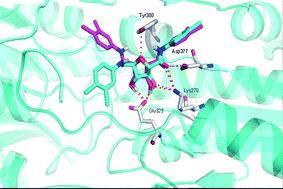 Human African trypanosomiasis, also commonly known as sleeping sickness, is a disease that leads to many deaths worldwide and is caused by protozoan parasites. When in the bloodstream these parasites can only produce the ATP (adenosine triphosphate) they need to survive via a glycolytic pathway, making this pathway essential for the parasite’s survival.
Human African trypanosomiasis, also commonly known as sleeping sickness, is a disease that leads to many deaths worldwide and is caused by protozoan parasites. When in the bloodstream these parasites can only produce the ATP (adenosine triphosphate) they need to survive via a glycolytic pathway, making this pathway essential for the parasite’s survival.