View the new videos on the Lab on a Chip YouTube site below:
A robust diffusion-based gradient generator for dynamic cell assays
View the new videos on the Lab on a Chip YouTube site below:
A robust diffusion-based gradient generator for dynamic cell assays
Nominations for the 2012 RSC Prizes and Awards close on the 15 January 2012
 Our Prizes and Awards represent the dedication and outstanding achievements and are a platform to showcase inspiring science to gain the recognition deserved. Don’t forget to nominate colleagues who have made a significant contribution to advancing the chemical sciences.
Our Prizes and Awards represent the dedication and outstanding achievements and are a platform to showcase inspiring science to gain the recognition deserved. Don’t forget to nominate colleagues who have made a significant contribution to advancing the chemical sciences.
View our full list of Prizes and Awards and use the online system to nominate a colleague.
Welcome to the first 2012 issue of Lab on a Chip
 On the front cover of our first issue of Volume 12 an the article from Neus Sabaté et al. on their fuel cell-powered microfluidic platform for lab-on-a-chip applications. This hot article was recently highlighted in Chemistry World.
On the front cover of our first issue of Volume 12 an the article from Neus Sabaté et al. on their fuel cell-powered microfluidic platform for lab-on-a-chip applications. This hot article was recently highlighted in Chemistry World.
Fuel cell-powered microfluidic platform for lab-on-a-chip applications
Juan Pablo Esquivel, Marc Castellarnau, Tobias Senn, Bernd Löchel, Josep Samitier and Neus Sabaté
DOI: 10.1039/C1LC20426B
 On the inside front cover we have an image from Eric Stava et al. showing their work on the mechanical actuation of ion channels using a piezoelectric planar patch clamp system.
On the inside front cover we have an image from Eric Stava et al. showing their work on the mechanical actuation of ion channels using a piezoelectric planar patch clamp system.
Mechanical actuation of ion channels using a piezoelectric planar patch clamp system
Eric Stava, Minrui Yu, Hyun Cheol Shin, Hyuncheol Shin, Jonathan Rodriguez and Robert H. Blick
DOI: 10.1039/C1LC20636B
In this issue we also have the editorial introduction from Editor Harp Minhas – Meeting the challenge – discussing our new developments and plans for the coming year, we think it’s going to be an exciting one!
Microfluidic devices in bioanalysis offer advantages in terms of high-throuphput and reduced costs per analysis. In this paper James Rusling and colleagues at the University of Connecticut use gold compact disks in the construction of inexpensive immunomicroarrays. They then used their devices for the electrochemical detection of the cancer biomarker, interleukin-6 in diluted serum.
Find out the details and read the article – Free for 4 weeks 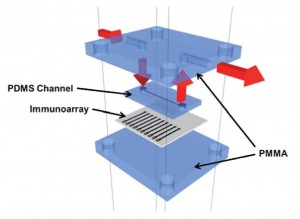
Fabrication of immunosensor microwell arrays from gold compact discs for detection of cancer biomarker proteins
Chi K. Tang, Abhay Vaze and James F. Rusling
Lab Chip, 2012, Advance Article
DOI: 10.1039/C1LC20833K, Paper
This month sees the following articles in Lab on a Chip that are in the top ten most accessed:
Rapid prototyping polymers for microfluidic devices and high pressure injections
Elodie Sollier, Coleman Murray, Pietro Maoddi and Dino Di Carlo
Lab Chip, 2011, 11, 3752-3765
DOI: 10.1039/C1LC20514E
Droplet microfluidics—a tool for protein engineering and analysis
Haakan N. Joensson and Helene Andersson-Svahn
Lab Chip, 2011, 11, 4144-4147
DOI: 10.1039/C1LC90102H
Surfactants in droplet-based microfluidics
Jean-Christophe Baret
Lab Chip, 2012, Advance Article
DOI: 10.1039/C1LC20582J
Bubbles navigating through networks of microchannels
Wonjae Choi, Michinao Hashimoto, Audrey K. Ellerbee, Xin Chen, Kyle J. M. Bishop, Piotr Garstecki, Howard A. Stone and George M. Whitesides
Lab Chip, 2011, 11, 3970-3978
DOI: 10.1039/C1LC20444K
Integration of intra- and extravasation in one cell-based microfluidic chip for the study of cancer metastasis
Min Kyeong Shin, Sung Kyu Kim and Hyungil Jung
Lab Chip, 2011, 11, 3880-3887
DOI: 10.1039/C1LC20671K
Microfluidic static droplet arrays with tuneable gradients in material composition
Meng Sun, Swastika S. Bithi and Siva A. Vanapalli
Lab Chip, 2011, 11, 3949-3952
DOI: 10.1039/C1LC20709A
Controlled viable release of selectively captured label-free cells in microchannels
Umut Atakan Gurkan, Tarini Anand, Huseyin Tas, David Elkan, Altug Akay, Hasan Onur Keles and Utkan Demirci
Lab Chip, 2011, 11, 3979-3989
DOI: 10.1039/C1LC20487D
Droplet formation via flow-through microdevices in Raman and surface enhanced Raman spectroscopy—concepts and applications
Anne März, Thomas Henkel, Dana Cialla, Michael Schmitt and Jürgen Popp
Lab Chip, 2011, 11, 3584-3592
DOI: 10.1039/C1LC20638A
1-Million droplet array with wide-field fluorescence imaging for digital PCR
Andrew C. Hatch, Jeffrey S. Fisher, Armando R. Tovar, Albert T. Hsieh, Robert Lin, Stephen L. Pentoney, David L. Yang and Abraham P. Lee
Lab Chip, 2011, 11, 3838-3845
DOI: 10.1039/C1LC20561G
Large-scale plasmonic microarrays for label-free high-throughput screening
Tsung-Yao Chang, Min Huang, Ahmet Ali Yanik, Hsin-Yu Tsai, Peng Shi, Serap Aksu, Mehmet Fatih Yanik and Hatice Altug
Lab Chip, 2011, 11, 3596-3602
DOI: 10.1039/C1LC20475K
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Lab on a Chip? Then why not submit to us today or alternatively email us your suggestions.
Welcome to the final issue of 2011!
 On the front cover of Issue 24 we have an article from Per Augustsson et al., who have developed a platform for micro particle image velocimetry (μPIV) for analyzing two-dimensional acoustophoresis. The device is automated, temperature-stable and has uncertainties below 5% and is therefore able to conduct high-precision measurement of the acoustophoretic velocity field in microchannels.
On the front cover of Issue 24 we have an article from Per Augustsson et al., who have developed a platform for micro particle image velocimetry (μPIV) for analyzing two-dimensional acoustophoresis. The device is automated, temperature-stable and has uncertainties below 5% and is therefore able to conduct high-precision measurement of the acoustophoretic velocity field in microchannels.
Automated and temperature-controlled micro-PIV measurements enabling long-term-stable microchannel acoustophoresis characterization
Per Augustsson, Rune Barnkob, Steven T. Wereley, Henrik Bruus and Thomas Laurell
DOI: 10.1039/C1LC20637K
 The inside front cover highlights the article from Kevin Kit Parker and colleagues that recently featured in Chemistry World. The article describes a ‘heart on a chip’, exploiting muscular thin film technology to measure contractility and the effect of cell architecture on tissue contraction.
The inside front cover highlights the article from Kevin Kit Parker and colleagues that recently featured in Chemistry World. The article describes a ‘heart on a chip’, exploiting muscular thin film technology to measure contractility and the effect of cell architecture on tissue contraction.
Ensembles of engineered cardiac tissues for physiological and pharmacological study: Heart on a chip
Anna Grosberg, Patrick W. Alford, Megan L. McCain and Kevin Kit Parker
DOI: 10.1039/C1LC20557A
Also in this issue is the latest Research Highlights article from Ali Khademhosseini, and a Focus article on droplet microfluidics for protein engineering and analysis from Helene Andersson Svahn and Haakan Joensson.
 A Lab on a Chip article from Scott Manalis, MIT, and colleagues has been featured in the New Scientist! The article describes methods for trapping single cells and monitoring their response to drugs within a suspended microchannel resonator.
A Lab on a Chip article from Scott Manalis, MIT, and colleagues has been featured in the New Scientist! The article describes methods for trapping single cells and monitoring their response to drugs within a suspended microchannel resonator.
By measuring changes in cell size and growth when drugs are introduced to the micromechanical system Manalis hopes that we will eventually be able to predict whether cancer treatments will be effective for individual patients, ‘we plan to determine if the growth response of tumour cells can be predictive of how a patient will respond to a therapy’.
To learn more read the article in the New Scientist – ‘Microscopic scales weigh up cancer therapies‘ – or go straight to the research article:
Mass sensors with mechanical traps for weighing single cells in different fluids
Yaochung Weng, Francisco Feijó Delgado, Sungmin Son, Thomas P. Burg, Steven C. Wasserman and Scott R. Manalis
DOI: 10.1039/C1LC20736A
View the new videos on the Lab on a Chip YouTube site below:
Single- and two-phase flow in microfluidic porous media analogs based on Voronoi tessellation
View the new videos on the Lab on a Chip YouTube site below:
Planar silicon microrings as wavelength-multiplexed optical traps for storing and sensing particles
Converting steady laminar flow to oscillatory flow through a hydroelasticity approach at microscales
Lateral cavity acoustic transducer as an on-chip cell/particle microfluidic switch
Malaysian scientists have created a flexible and environmentally friendly microfluidic device using a cloth decorating technique for printing wax onto cotton.
Dedy Wicaksono at the University of Technology Malaysia was inspired by his batik-patterned clothes to create the device. ‘Batik processing is wax patterning to create regions of differing hydrophilicity and hydrophobicity on cloth,’ says Dedy. The technique is traditionally used to prevent dye spreading from one area of cloth to another, creating coloured patterns. Using wax printing methods for paper and silk based microfluidic devices is known, but these have required specialist equipment or expensive materials.
Wicaksono’s method needs neither of these things. First, his team prepared the cotton by scouring it with sodium hydroxide and anhydrous sodium carbonate solutions. The treatment removes the outer layer, exposing underlying cellulose fibres. Both the chemical composition (increased oxygen content) and physical structure (increased surface roughness) of the fibre surface were altered, increasing the wettability and wicking rate.
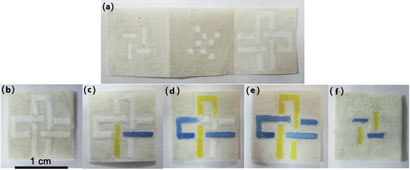
(a)-(f) are photographs showing two hydrophilic channels with different dye solutions that cross each other vertically and horizontally in three layers without mixing. (b)-(e) are front views of the device before, 5 seconds, 2 minutes and 5 minutes after dropping the dyes into different channels. (f) is the bottom layer 5 minutes after adding the dyes.
Then, they printed the pattern for the microfluidic device onto paper. The paper was dipped into hot batik wax, then dried, before the pattern was cut from the sheet and attached to the scoured cloth using a few pins. Heat treatment melted the wax again, and it spread onto the surface and into the cloth, filling the gaps in the weave and within the fibres. The fatty acids in the wax increase the hydrophobicity of the fibres where they are applied, creating barriers to liquid flow.
Dedy’s group made both 2D and 3D devices; the latter was made by folding layers of the patterned cloth. A test using ink solutions revealed the ink moving along the cloth’s hydrophilic channels, filling a device in minutes. In a further test, the team was able to detect the protein bovine serum albumin colorimetrically using the devices, with the result visible to the naked eye.
‘A key innovation here is the batik-inspired method of transferring patterned wax on paper to cotton cloth,’ comments Shashi Murthy, an expert in microfluidic devices at Northeastern University, US. He adds that the technology ‘has the potential to provide a rapid and low cost readout for analytes characterised by relatively simple colorimetric assays’.
Dedy is now investigating ways to exert more control over the liquid flow so that more complex microfluidic devices can be developed. ‘By making the channels inside a flexible cloth, we are envisioning an embeddable wearable lab in the very near future,’ he says.
Flexible microfluidic cloth-based analytical devices using a low-cost wax patterning technique
Azadeh Nilghaz, Dedy H. B. Wicaksono, Dwi Gustiono, Fadzilah Adibah Abdul Majid, Eko Supriyanto and Mohammed Rafiq Abdul Kadir
Lab Chip, 2012, Advance Article
DOI: 10.1039/C1LC20764D
Original article published at Chemistry World