New YouTube Videos
New microfluidic system for intracochlear drug delivery
an article by Claire Weston, PhD student at Imperial College London
William Sewell at Massachusetts Eye and Ear Infirmary and Harvard Medical School and Jeffrey Borenstein at the Charles Stark Draper Laboratory in Massachusetts have developed an automated micropump device for direct delivery of drugs into the perilymph fluid within the cochlea. This has potential for use in the treatment of sensorineural hearing loss and would remove the toxicity issues that are common when drugs are administered systemically. This is one of the most common forms of hearing loss and is caused by damage to the sensory hair cells or to the auditory nerve.
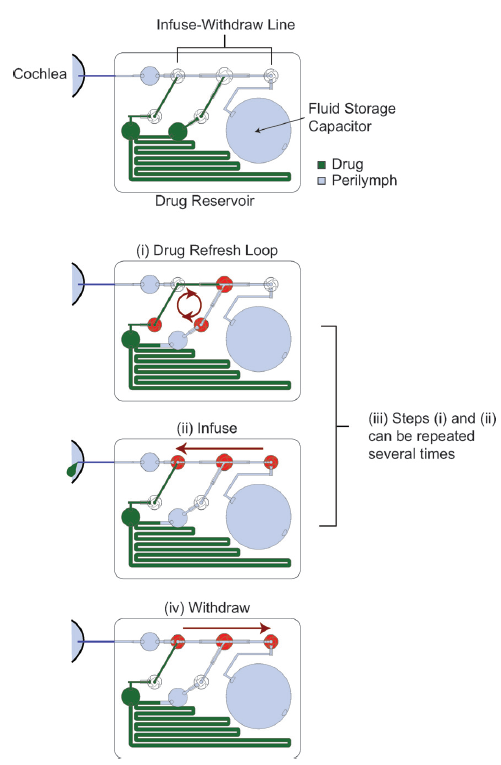
Due to the small volumes of perilymph fluid within the cochlea (~ 0.2 mL) and the sensitivity of the ear, the authors have developed a reciprocating delivery system, where an accurate volume of the concentrated drug can be infused and, once given time to distribute, the same volume of fluid can be withdrawn, resulting in zero overall net increase in cochlear fluid. The specific design also minimised the dead volume present in the device in order to reduce the amount of pumping needed, and by incorporating capacitors, prevented high flow rates during pumping, which can lead to cochlear damage.
The authors emphasise the need for a device that is small and lightweight enough to be implanted near to the cochlea and that is also able to administer precise sub-microliter volumes of fluid over several days or months. The microfluidic device presented in Lab on a Chip has been fabricated onto a ~4 x 3 cm chip and is capable of delivering accurate and repeatable volumes of fluid over more than 1000 pump strokes. The authors highlight that by incorporating the device onto a head mount, this particular design could be used in animal models for preclinical drug characterisation, where extensive studies are required.
All the fluidic components of this system have been incorporated into the chip, so that, if battery operated, it could be used as a stand-alone device. In this design, a separate controller was used; however, it is stated that the control circuitry could also be miniaturised and incorporated into the chip, for use with a battery. Efforts were also made to minimise the power consumption of the pump for this purpose. The main components of the device are a drug reservoir, a fluid storage capacitor which contains artificial perilymph for flushing the system, an infuse-withdraw line, and multiple valves to control the different steps of the drug delivery process, as shown in the diagram.
Dose control was successfully demonstrated by loading the pump with fluorescein as the test drug and monitoring the fluorescence of the aliquots collected following different dosage schemes. Several studies were also carried out on guinea pigs using a glutamate receptor antagonist as the test drug. This compound reversibly suppresses compound action potentials (CAPs) in the cochlea – monitoring changes in CAP amplitude and threshold can be used to test for hearing loss.
The results showed that fully reversible hearing loss was induced and this was used to estimate the optimum wait time between infusion and withdrawal for the reciprocating delivery. The distribution of the drug in the ear was also monitored by measuring changes to CAPs at different frequencies and comparing these to the known tonotopic organisation of the cochlea. To test for cochlear damage, the authors monitored another hearing response (distortion product otoacoustic emission) that was not expected to change, and determined that there was no acute mechanical damage.
This drug delivery system has excellent potential for use in clinical and preclinical trials and also for long term treatment of hearing loss using existing drugs. The potential for battery operation is particularly important, and is an aspect that the authors are now focusing on for future work.
To download the full article for free* click the link below:
Microfabricated reciprocating micropump for intracochlear drug delivery with integrated drug/fluid storage and electronically controlled dosing
Vishal Tandon, Woo Seok Kang, Tremaan A. Robbins, Abigail J. Spencer, Ernest S. Kim, Michael J. McKenna, Sharon G. Kujawa, Jason Fiering, Erin E. L. Pararas, Mark J. Mescher, William F. Sewell, Jeffrey T. Borenstein
Lab Chip, 2016, 16, 829-846
DOI: 10.1039/C5LC01396H
—————-

About the webwriter
Claire Weston is a PhD student in the Fuchter Group, at Imperial College London. Her work is focused on developing novel photoswitches and photoswitchable inhibitors.
—————-
*Access is free until 05/04/2016 through a registered RSC account.
Cleaning solution test doesn’t move contact lens wearers to tears
A Lab on a Chip article highlighted in Chemistry World article by Harriet Brewerton
In the US, over 30 million people wear contact lenses and each year around 60,000 contract serious eye infections that put them at risk of going blind. Thorough cleaning is vital to prevent bacterial build-up on the lens but research has also shown that an individual’s tear chemistry affects the effectiveness of cleaning solutions.
Personalised care products can prevent serious eye infections caused by ineffective contact lens cleaning solutions
The team use their device to demonstrate how tears impacts lens selection and care, and say it could be adapted for point-of-care testing in eye clinics.
Please visit Chemistry World to read the full article.
Contact lens-on-a-chip companion diagnostic for personalized medicine
Allan Guan, Yi Wang, Kenneth Scott Phillips and Zhenyu Li
Lab Chip, 2016, Accepted Manuscript
DOI: 10.1039/C6LC00034G
*Access is free through a registered RSC account until 8th April 2016 – click here to register
New YouTube Videos
New YouTube Videos
Optimising the conditions for biocrude formation using microfluidics
an article by Claire Weston, PhD student at Imperial College London
If the Lab on a Chip HOT articles are anything to go by, using microalgae as a feedstock for biofuel is definitely a flourishing research area. Microalgae is a particularly attractive feedstock as it grows rapidly, has a large oil content, and can be grown pretty much anywhere.
David Sinton and co-workers at the University of Toronto have previously published in Lab on a Chip on this topic and have now reported their work on optimising the conditions for converting microalgae ‘biomass’ into crude biofuel (‘biocrude’). The process by which this is achieved is known as hydrothermal liquefaction. High temperatures and pressures are employed to break down the organic compounds from the biomass into the oils that make up biocrude.
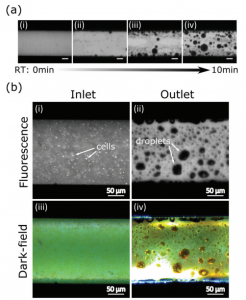
a) Fluorescence images at increasing reaction time; b) Fluorescence and dark-field imaging of fluids at inlet and outlet.
The Sinton lab have developed a microfluidic chip in order to accurately control the reaction conditions of this process and also to study the effect of changing conditions on the biofuel that is formed. The continuous flow and small volume of the chip allow very fast heating of the algal slurry so reaction times can be accurately studied – in fact the heating rate achieved is the fastest reported to date. The slurry was analysed in situ by fluorescence imaging and changes to the fluorescence signature were monitored. Over the course of the reaction, the fluorescence signal due to chlorophyll disappeared and a new peak developed, indicating the formation of the aromatic compounds that are a characteristic component of crude oil and plant based oils.
Further analysis of the samples collected from the chip outlet found that the energy content (measured by the elemental composition) of the biocrude reached saturation after short reaction times – much before the fluorescence signal stopped changing. In addition to this, non-fluorescent droplets could be seen inside the reaction chamber, as shown in the diagram on the left, which were presumed to comprise of aliphatic oils. These findings indicate that analysis of the elemental composition alone is insufficient to measure chemical conversion to biocrude and methods such as fluorescence imaging should also be employed.
This work is the first example of using a microfluidic platform in hydrothermal liquefaction research and just goes to highlight the versatility of lab-on-a-chip systems.
To download the full article for free* click the link below:
Biomass-to-biocrude on a chip via hydrothermal liquefaction of algae
Xiang Cheng, Matthew D. Ooms and David Sinton
Lab Chip, 2016, 16, 256-260
DOI: 10.1039/C5LC01369K
—————-

About the webwriter
Claire Weston is a PhD student in the Fuchter Group, at Imperial College London. Her work is focused on developing novel photoswitches and photoswitchable inhibitors.
—————-
*Access is free through a registered RSC account until 29/02/2016.
Microsystems based diagnostics: new developments and novel tools
Reported by Shavon Kumar, CSIR, South Africa
The third international Micro-Med-A workshop took place in Stellenbosch, South Africa, from 16-19 September 2015. The workshop provided the perfect platform to bring together leaders in the field of microsystems technologies as well as industry partners and medical experts to discuss new ideas and strategies to develop cutting edge point-of-care (PoC) diagnostics to address solutions for real world problems by building a collaborative network across various disciplines and by crossing geographical borders.
The theme for the workshop was the development of rapid point-of-care technologies for various applications relating to health and the environment.
The workshop opened with a proposal put forth by the chairmen, Kevin Land from the Council for Scientific and Industrial Research (CSIR) in South Africa and Jan Korvink from Karlsruhe Institute of Technology (KIT) in Germany. It was suggested that the outcome of the meeting should be the collaborative effort of experts in various fields working on a single extreme PoC project with combined expertise and combined resources, where each group would focus on an aspect of the bigger project with the common goal of addressing one defined problem.
The conference started with presentations from clinicians and pathologists whose daily work involves diagnostic testing and interactions with patients for whom rapid PoC tests would ultimately be aimed. Point of care is defined as a low cost, rapid diagnostic test or service that can be completed at the point of testing, independent of a centralised high infrastructure laboratory. In 2014 the recorded population of South Africans living in rural settings was 35.7%. Such settings do not have the infrastructure for high-tech laboratory and medical care facilities. In many instances visiting a clinic for medical attention comes at a cost of a day’s wage while also incurring travelling expenses. Furthermore, many people do not make the required follow up visits to receive results or treatment. Therefore PoC tests are well suited to the African landscape where clinics and medical facilities are far from rural communities.
The event presented work by industry partners and representatives from commercial companies which provided a fresh outlook on collaborative networks. This bridged the gap between academics and industry where the latter can serve to provide well established platforms that can be integrated into developed or developing technology without re-inventing the wheel. This provides a twofold advantage, mainly reduction to development costs and time to market.
Paper based microfluidic devices or µPADs are an attractive platform for diagnostic tests. Paper is cheap, easily accessible and easily printed on; it can be burnt and therefore there is no need for costly biohazard waste disposable facilities. Paper is easily stacked making it easy to transport and therefore deliverable to end users. Furthermore, paper is self-wetting and does not require instrumentation for readout. Therefore, paper based tests are gaining more recognition as the solution to PoC tests as it meets many of the ASSURED criteria for rapid PoC. Needless to say, there are many research groups developing PoC tests using paper substrates and this was showcased at the workshop. Some of the technologies presented at the Micro-Med-A workshop showed paper based PoC applications in the developmental stage of research for the detection of toxic metals and bacterial detection in the environment. This further emphasised that paper based tests can address an important niche in diagnostics.
The workshop fostered an environment for excitement in the field of microsystems for African health through many interactive discussions and insights from participants of various backgrounds. The workshop enabled new networks to be established, while strengthening existing ones, and mapped the overall bigger picture of what is required to address health issues, particularly in under-resourced settings such as those in rural Africa and India.
The workshop closed with many suggestions from delegates for future meetings with the groundwork being laid for collaborative efforts. Information on this workshop can be found at www.micromed2015.co.za. The next workshop will be held in September 2017. People interested in receiving details once they are available should contact Kevin Land.
Delegates who attended the MicroMed 2015 workshop in South Africa.