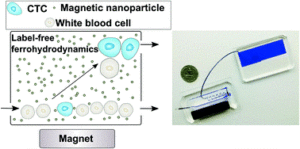
We are delighted to introduce our latest Lab on a Chip Emerging Investigator, Wilbur Lam!
Wilbur A. Lam, MD, PhD is an Associate Professor of Biomedical Engineering and Pediatrics at the Georgia Institute of Technology and Emory University and has a unique background as a physician-scientist-engineer trained in pediatric hematology/oncology and bioengineering. Dr. Lam’s interdisciplinary laboratory, located at both Emory and Georgia Tech, includes engineers, biologists, biophysicists, chemists and physicians. Our laboratory serves as a unique “one-stop shop” in which we develop in vitro microsystems to study hematologic processes in both health and disease and then immediately bring those technologies to the patient bedside. More specifically, the Lam laboratory’s research interests involve the development and application of microsystems to enable research in pathologic biophysical blood cell interactions that occur in diseases such as sickle cell disease and thrombosis, as well as further translating those systems into novel therapeutics and diagnostic devices.
Read Wilbur’s Emerging Investigator series paper “Probing blood cell mechanics of hematologic processes at the single micron level” and find out more about him in the interview below:
Your recent Emerging Investigator Series paper focuses on probing blood cell mechanics of hematologic processes. How has your research evolved from your first article to this most recent article?
As a bioengineer and a physician specializing in paediatric haematology, my initial goals for our lab were really twofold: 1) to leverage microscale technologies to apply cell mechanics principles towards investigating clinically relevant biologic processes and 2) to convince the medical and clinical haematology fields that physical phenomena such as shear stress and the mechanical properties of the microenvironment can directly mediate the biologic processes of blood cells and pathophysiology of blood diseases such as sickle cell disease and thrombosis. Over the last few years, I think our lab and other groups have accomplished that and we’re now concentrating on not only the basic science questions of blood cell mechanics but how to apply the microtechnologies we develop as potential diagnostics and even therapeutic systems for patients with blood diseases.
What aspect of your work are you most excited about at the moment?
Along those same lines, we’ve been at this game long enough to see some of our microtechnologies translate to my patients with blood diseases, which we find pretty exciting. For example, we just received FDA clearance for a point-of-care anaemia diagnostic our lab developed. We also have several other blood cell mechanics-based microtechnologies that are in our lab’s translational pipeline we’re pretty excited about. For instance, we’ve developed a microfluidic system that can assess platelet contraction forces at the single cell level and we’re now trying to determine whether that system can be used to diagnose patients with bleeding disorders, a clinical interest of mine. At the same time, we’re also trying to leverage that mechanical phenomenon of platelet contraction as a “Trojan Horse” strategy of targeted drug delivery for haemophilia, which we find to be pretty sci-fi and exciting. Moreover, using fairly simple microfluidic devices, we determined that intravenous hydration, a standard first-line therapy for sickle cell disease, may have beneficial as well as deleterious effects on red cell mechanics, which could potentially alter how we treat this disease. We’ve also developed technologies that can enable the translation of other technologies including a microfluidic device that can improve the transduction efficiency of lentiviruses for gene therapy and CAR T-cell therapy, which are both making significant clinical impacts right now. That said, we still remain very interested in the basic science of blood cell mechanics, which is evidenced by this current paper of ours, and we have active projects including but not limited to investigating leukocyte mechanics and are developing new microfluidic strategies to study and model bleeding.
In your opinion, what is the biggest potential impact the results of this research will have on blood disorder diagnostics?
I think impact of our research regarding blood disorder diagnostics can be measured in several ways. First, we obviously want to positively affect and help as many patients and people as possible, which is why we’re clearly excited about the recent FDA 510K clearance of our point-of-care anaemia diagnostic. For that project, we’re currently planning our manufacturing and distribution strategy with different partners to commercialize and disseminate this technology in the US and abroad to impact as many people globally as we can. In addition, we can also measure impact on a more personal level. One of the reasons why our lab has been fortunate enough to be able to recruit the best graduate students and postdoctoral fellows is because of our unique setup in which our lab is located both on the engineering side at Georgia Tech as well as the clinical side at Emory University and Children’s Healthcare of Atlanta, where I am a practicing physician. I’ve truly been blessed to work with the best and most talented students and postdocs in the field of bioengineering and I think they enjoy the fact that they can design a device and literally see it be used on a real live patient within a relatively short time frame. This is great for my patients as well, who are able to witness firsthand how medical technology develops and the potential of how it can improve their own lives in the near future. So, in essence, our lab has developed a “basement-to-bench-to-bedside” approach in which we design and develop microtechnologies to not only study disease but also directly translate these to the patients I care for and even conduct clinical assessments and trials for those devices – all under one roof. By the way, if your readers can help me come up with a better word than “basement” while still maintaining the alliteration as well as the overall message of the phrase, my lab and I will be extremely grateful.
What do you find most challenging about your research?
Like many other bioengineering laboratories among your readership, successful design and development of a novel micro/nanoscale technology is only half the battle. Then you have to do the actual experiment to demonstrate its utility and hope it delivers some type of value. This can be frustrating at times, as our projects can take twice as long as average, but the hope is that our impact will be doubled as well.
In which upcoming conferences or events may our readers meet you?
I attend the annual American Society of Hematology and International Society on Thrombosis and Haemostasis meetings as I serve on the scientific committees for both of them. I also frequently attend the annual Biomedical Engineering Society and MicroTAS meetings as well.
How do you spend your spare time?
I answer interview questions for scientific blogs! Seriously, I’m a huge fan of pop culture and pop music. I even have a lifetime subscription to Rolling Stone, which I’ve been starting to regret in recent years as I’ve grown older as the covers of that magazine have progressively gotten more risqué (or maybe that’s just my perception as whatever semblance of hipness and coolness I ever had is exponentially decreasing with time). I also still pick up my guitar every now and then – especially when I’m procrastinating writing a grant or submitting grades.
Which profession would you choose if you were not a scientist?
I find that when most people are asked this question, they give an idealized answer, so I’ll do the same. I’d like to say that I’d be a successful songwriter/musician as I’ve played guitar in bands from my teenage years until my first faculty position (during my clinical training, I played in an all-paediatrician band named “Booster Shot”). However, to be frank, I really wasn’t that good and seriously doubt I could have made it as a professional musician. So, I think the realistic answer is that my most likely alternative profession would be that of a disgruntled sales associate at a Guitar Center somewhere or working as a roadie for artists a fraction of my age and whom I most likely would have despised. So, it’s good that this science and medicine thing worked out…
Can you share one piece of career-related advice or wisdom with other early career scientists?
Hone your writing skills, start early and keep practicing. I am constantly amazed at how much of my time is devoted to writing and how important that skill is to be a successful scientist. Whether it’s writing or editing papers, lectures, or grants, I find I actually spend most of my time writing. In retrospect, this makes sense as communication really is the currency of science, but there’s no way my younger self would believe me if I were to travel back in time to let him what was ahead of him. I’ve had many young people tell me they want to go into science specifically because they dislike writing, which is obviously a misperception I’m quick to point out. In fact, one joke (and not a very good one, admittedly) I often share with my students is that as a scientist, I actually write really bland non-fiction for a living and for a very small audience, and when I’m grantwriting, I’m only writing to an audience of three people.
Comments Off on Emerging Investigator Series – Wilbur Lam