We are delighted to introduce our latest Lab on a Chip Emerging Investigator – Ian Wong!
Ian Y. Wong is currently an Assistant Professor of Engineering and of Medical Science at Brown University. He completed an A.B. in Applied Mathematics from Harvard University in 2003, Ph.D. in Materials Science and Engineering with Nick Melosh at Stanford University in 2010, and postdoctoral training at Massachusetts General Hospital with Mehmet Toner and Daniel Irimia in 2013. He has been recognized with an NSF Graduate Research Fellowship, a Damon Runyon Cancer Research Fellowship, the Brown University Pierrepont Prize for Outstanding Advising, as well as a Biomaterials Science Emerging Investigator. His research interests include the development of miniaturized technologies to investigate cancer cell invasion, phenotypic plasticity and drug resistance. Moreover, his group engineers unconventional fabrication techniques for printing and patterning nano/bio materials.
Read his Emerging Investigators paper “Stereolithographic printing of ionically-crosslinked alginate hydrogels for degradable biomaterials and microfluidics“, watch the associated video and find out more about his research in the interview below:
Your recent Emerging Investigator Series paper focuses on stereolithographic printing of ionically-crosslinked alginate hydrogels. How has your research evolved from your first article to this most recent article?
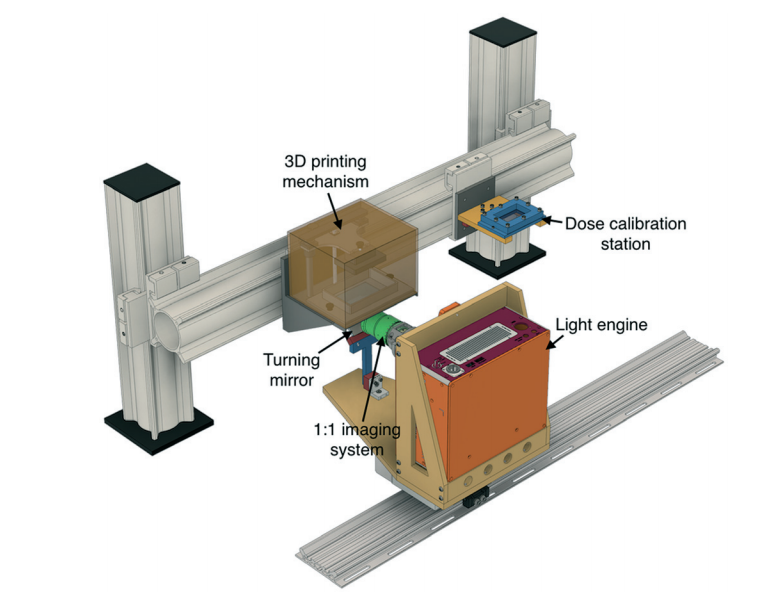
I give complete credit to my graduate student, Tom Valentin, who came up with this approach to light-based 3D printing via ionic crosslinking – and then actually got it to work. In retrospect, my Ph.D. thesis focused on biomolecular self-assembly based on ionic interactions, so it’s serendipitous that my current research has circled back to some of these concepts.
What aspect of your work are you most excited about at the moment?
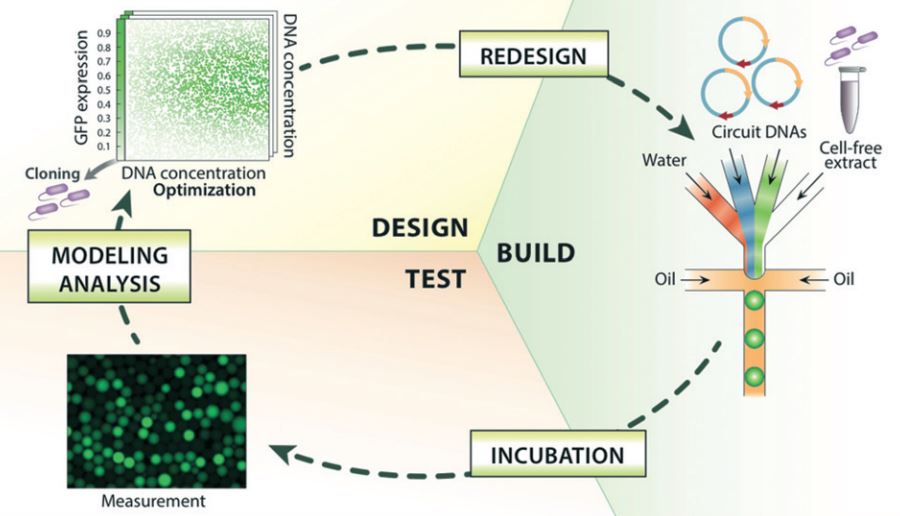
My lab integrates biomaterials, microfluidics, and computer vision to investigate cancer cell migration and drug resistance. Our first few papers set down the foundations for these different technologies, but now we’re starting to put these pieces together to gain some fascinating insights into cancer biology.
In your opinion, what is the biggest advantage of stereolithographic printing of hydrogels over other printing techniques?
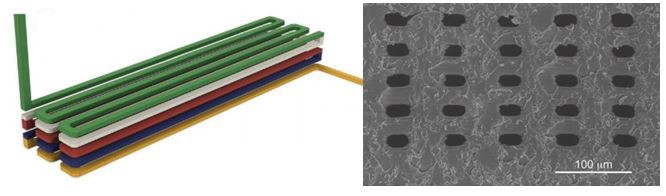
Conventional light-based 3D printing of soft materials is based on covalent crosslinking, which results in strong but irreversible bonds. Our demonstration of light-based patterning using reversible ionic crosslinks should enable smart and “biomimetic” properties such as self-healing and stimuli-responsiveness. These properties have been previously demonstrated in bulk hydrogels, but remain relatively nascent for 3D printed structures.
What do you find most challenging about your research?
I work at the interface of engineering and cancer biology, and I find that it takes a lot of effort to bridge between these two communities and become fluent in both disciplines. Moreover, there are twice as many things that can go wrong with the experiments! Nevertheless, it has been extremely worthwhile to see how our technologies could potentially make an immediate and highly meaningful impact.
In which upcoming conferences or events may our readers meet you?
I will be attending BMES this October in Phoenix, AZ.
How do you spend your spare time?
Whenever possible, I enjoy dining out with my wife. I also enjoy cycling, which helps to burn off all those calories
Which profession would you choose if you were not a scientist?
I’ve always been interested in entrepreneurship, and this is something I will likely revisit once my lab and technologies become more established.
Can you share one piece of career-related advice or wisdom with other early career scientists?
Early career scientists are constantly pulled in many directions and have limited time to commit to anything. Nevertheless, I try my best to spend a lot of time with my students and postdocs early on. Such mentoring helps trainees transition towards independence and can also catch problems before they become serious, so it is incredibly worthwhile in the long run.