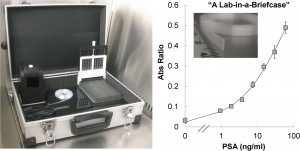
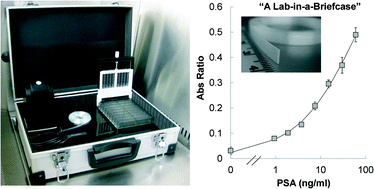
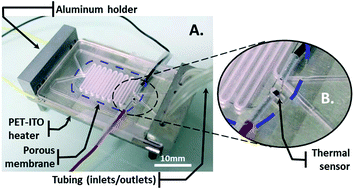
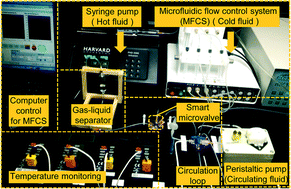
In a recent paper in Lab on a Chip, a group of British researchers reported a ‘lab-in-a-briefcase’ for detection and quantification of the prostate cancer biomarker PSA in human serum and whole blood. Their lab-in-a-briefcase is a small container with a set of coated plastic capillaries, a pre-loaded microwell plate with reagents and a film scanner.
The researchers stress that their system is cheap and easy to handle, which would make it very useful for performing diagnostics in low resource areas. In addition, their lab-in-a-briefcase demonstrates the potential for point-of-care tests for prostate cancer, which would allow easy screening by non-experts in a non-clinical setting.
The concept of a lab-in-a-briefcase may have more far-reaching implications, though. Most lab-on-a-chip assays and microfluidic systems are usually developed in the context of interdisciplinary research collaborations. One research department may develop a new system, while another department has the – often unstable – samples that are used to demonstrate proof-of-concept. This complexity means that projects can quickly become logistic nightmares.
Multi-site collaborations make the portability and the standardized format that are found in the lab-in-a-briefcase and related technologies very important. It doesn’t matter if the application domain of a project is physics, biochemistry or biology. Developing a portable, standardized set-up with good documentation, automated analysis and easy read-out can contribute greatly to the success of a multi-disciplinary microfluidic engineering project, because it promotes collaboration and a wider application of the technology early on.
All of this means that the lab-in-a-briefcase is not just a niche product that is only useful for cheap point-of-care diagnostics in low resource areas. It is a design concept that anyone in the realm of microfluidic engineering needs to understand. Perhaps the concept is also applicable to the project you’re currently working on?
Go check it out for yourselves – you can download this paper fro free* for a limited time only!