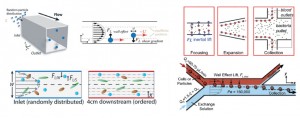
The Reynolds number, ratio of inertial to viscous forces, is low for most microfluidic platforms and has been approximated to zero to model most microscale fluid flows as linear and time-reversible (Stoke’s flow). Yet Dino Di Carlo’s group at the University of California- Los Angeles, among others, have shown that microfluidic systems in which Reynolds’ number ranges from 1 to 100 exhibit non-negligible inertial effects. These forces lead to separation and ordering of particles within channels by stream line crossing due to effects from the channel geometries (lift forces from the channel wall and velocity profile shear gradient). Inertial lift forces are regulated by the channel dimensions and geometry, particle diameter, and flow rate.[1]
Experimentally-derived intuitions have guided researchers to use the effects of inertial lift forces to produce high throughput flow cytometers to isolate bacteria from diluted blood samples,[2] systems capable of probing the deformability of cells to evaluate metastatic potential[3, 4] and platforms combined with Dean flow in curved channels to increase mixing of fluids or spirals to improve separation of particles.[5] Yet the physical underpinnings guiding these channel designs have been limited.
In this review, Amini and colleagues tackle many of the relationships which are important to create new devices by taking advantage of the unique contribution of inertial forces at the microscale. The behavior of non-Newtonian fluids (i.e., whole blood), the role of particle shape on focusing, particle-particle interactions, and the effect of protrusions along the channel length on flow (pillars, herringbone structures) are also discussed and can open exciting new applications in medical diagnostics, chemical synthesis, manufacturing of materials, and beyond. Inertial microfluidics platforms are en route to commercialization by Johnson & Johnson to sort rare circulating tumor cells from whole blood at 10 million cells per second (CTC-iChip[6]).
Download the full review for free* for a limited time only!
Inertial microfluidic physics
Hamed Amini, Wonhee Lee and Dino Di Carlo. Lab on a Chip, 2014, 14, 2739-2761.
DOI: 10.1039/C4LC00128A
References:
[1] D. Di Carlo, Lab on a Chip, 2009, 9, 3038-3046.
[2] A. J. Mach and D. Di Carlo, Biotechnol. Bioeng., 2010, 107, 302-311.
[3] J. S. Dudani, et al, Lab on a Chip, 2013, 13, 3728-3734.
[4] S. C. Hur, et al, Lab on a Chip, 2011, 11, 912-920.
[5] J. M. Martel and M. Toner, Scientific Reports, 2013, 3.
[6] E. Ozkumur, et al, Sci. Transl. Med., 2013, 5, 179ra47.
*Access is free through a registered RSC account until 25th August 2014 – click here to register
About the WebWriter
Sasha is a PhD student in bioengineering working with Professor Beth Pruitt’s Microsystems lab at Stanford University. Her research focuses on evaluating relationships between cell geometry, intracellular structure, and force generation (contractility) in heart muscle cells. Outside the lab, Sasha enjoys hiking, kickboxing, and interactive science outreach.