Researchers at the University of Canterbury in New Zealand have created a platform measuring mechancial forces exerted by the microscopic worm C. elegans in locomotion. The platform can measure all of the important worm locomotion parameters in a single assay. The team has noted that the worm’s crawling behaviors and thrust forces correlate to the structure of their microenvironment as the worm adjusts its behavior via mechanical sensing of its surroundings.
 C. elegans are soil-dwelling worms made up of just 959 cells (adult hermaphrodite). The worms are 1 mm long and 80 μm in body diameter. In labs studying C. elegans, the worms crawl on agar plates in search of food (usually E. coli) by twisting their body in sinusoidal waves.1 The sense of touch is crucial to these nematodes: 6 touch receptor neurons (mechanoreceptor neurons) allow the animal to detect external mechanical feedback with the environment as well as internal forces.2
C. elegans are soil-dwelling worms made up of just 959 cells (adult hermaphrodite). The worms are 1 mm long and 80 μm in body diameter. In labs studying C. elegans, the worms crawl on agar plates in search of food (usually E. coli) by twisting their body in sinusoidal waves.1 The sense of touch is crucial to these nematodes: 6 touch receptor neurons (mechanoreceptor neurons) allow the animal to detect external mechanical feedback with the environment as well as internal forces.2
The group led by Maan M. Alkaisi of The MacDiarmid Institute, New Zealand, and Wenhui Wang now at Tsinghua University, China, developed micropillar arrays of various arrangements and dimensions to modify the worm’s microenvironment and measure forces exerted by the worm as it crawls on the substrate. The researchers were also able to quantify locomotion parameters such as speed, amplitude of sine wave, and wavelength. Their unique assay combines 3 key elements: variable substrate topology, worm thrust force measurements, and locomotive metrics. One version of the device is also compatible with different substrates by mounting pillars on top of migrating worms instead of allowing worms to migrate directly on the microstructures.
The group found that reduced pillar spacing caused wild-type worms to exert twice the force to crawl through the substrate, especially in a honeycomb pillar arrangement. The worms were also much slower at moving in the narrow pillar structures and the frequency of sine wave propagations correlated to resistivity of the physical environment. The platform presented in this study enables many exciting studies in C. elegans locomotion and touch response, especially as many genetic mutant strains are available with changes to the number of mechanoreceptor neurons and muscle arms. 3
References:
1. S. Berri, J. H. Boyle, M. Tassieri, I. A. Hope and N. Cohen, HFSP journal, 2009, 3, 186-193.
2. M. B. Goodman, in WormBook: the online review of C. elegans biology, NIH Public Access, 2006, available from http://www.ncbi.nlm.nih.gov/books/NBK20187/.http://www.wormbook.org/chapters/www_mechanosensation/mechanosensation.html.
3. C. I. Bargmann and I. Mori, in C. elegans II. 2nd edition., ed. D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess, Cold Spring Harbor Laboratory Press, NY, 1997, available from http://www.ncbi.nlm.nih.gov/books/NBK20187/.
On-chip analysis of C. elegans muscular forces and locomotion patterns in microstructured environments
Shazlina Johari, Volker Nock, Maan M. Alkaisi, and Wenhui Wang. Lab Chip, 2013, 13, 1699-1707.
DOI: 10.1039/c3lc41403e
Sasha is a PhD student at Stanford University working with Professor Beth Pruitt’s Microsystems Lab. Her research interests focus on designing microscale devices for studying cell mechanobiology and the biophysical underpinnings of cell-cell and cell-substrate interactions.











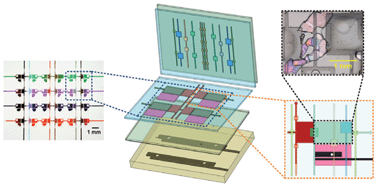
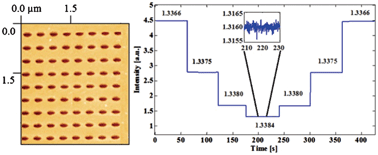
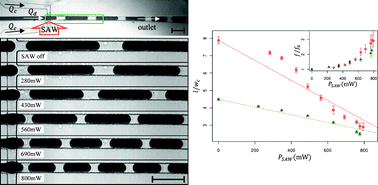

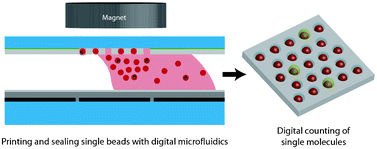
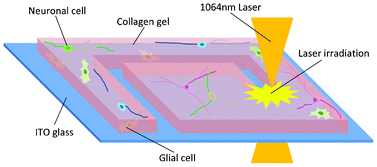
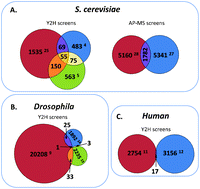
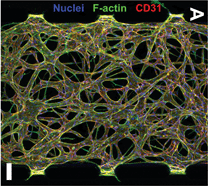









 Design concept (top) and operation mechanism (bottom) of the chip
Design concept (top) and operation mechanism (bottom) of the chip