Many diseases, especially cancers (and recurrences), are not detected until it is too late for effective treatment. Often, this is because available tests lack the sensitivity to find the appropriate protein biomarker in the body.1 Consequently, ultrasensitive tools for measuring proteins are vital for early diagnosis of diseases, and monitoring the effectiveness of surgery or therapy.
For 40 years, scientists and clinicians have been using immunoassays for protein detection. This technique relies on the precise recognition of a target molecule (antigen) by a unique Y-shaped protein (antibody) among thousands of interfering species. However, to achieve ultrasensitive detection, the antigen must be effectively transported to the antibody on the detection surface.2
To tackle this problem, a research team led by Prof. Martin Gijs drew inspiration from the human immune system. In the body, white blood cells are transported to the site of injury with the help of adhesion molecules. In the presence of blood flow, weak adhesion molecules cause the cells to slow down and roll along the vessel wall. This “rolling adhesion” allows the cells to search the wall meticulously for a stop signal. If they come across this signal, the cells will adhere firmly to the wall and squeeze into the site of injury.
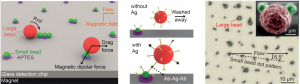
Taking cue from this phenomenon, the research team designed a microfluidic immunoassay using two sizes of magnetic beads coated with antibodies. First, ‘large’ (2.8 micrometer) beads are loaded with antigen (from the serum sample) in an on-chip mixing chamber. After unbound components are washed away, the large beads are flown over a surface decorated with a pattern of ‘small’ antibody-coated (1.0 micrometer) beads. In the presence of a magnet, the large beads will roll on the surface and interact closely with the small beads (via magnetic dipolar forces). Beads that are loaded with antigen will adhere firmly to the surface through antibody-antigen complexes, while beads with no antigen are washed away (via flow-induced drag forces).
The antigen concentration in the serum sample can be detected by simply counting the number of large beads in the detection area. This method can rapidly (<20 min) detect down to 200 proteins (Tumor necrosis factor-α) in a 5 microliter of sample (i.e. 60 attomolar), making it one of the fastest and most sensitive immunoassays ever reported. Such salient technique has the potential to improve treatment and outcome for cancer patients worldwide.
The above paper is part of our Lab on a Chip Top 10%, a collection of articles selected by the Editors at Lab on a Chip, from all our high quality papers, to be of exceptional significance for the miniaturisation community. Papers in this category will have received excellent reports during peer review, and demonstrate a breakthrough in device technology, methodology or demonstrate important new results for chemistry, physics, biology or bioengineering enabled by miniaturisation.
The full paper details are here: Attomolar protein detection using a magnetic bead surface coverage assay, H. Cumhur Tekin, Matteo Cornaglia and Martin A. M. Gijs*, Lab Chip, 2013, 13, 1053-1059. DOI: 10.1039/C3LC41285G
1. C. S. Thaxton, R. Elghanian, A. D. Thomas, S. I. Stoeva, J.-S. Lee, N. D. Smith, A. J. Schaeffer, H. Klocker, W. Horninger, G. Bartsch and C. A. Mirkin, Proceedings of the National Academy of Sciences, 2009, 106, 18437-18442.
2. T. M. Squires, R. J. Messinger and S. R. Manalis, Nature Biotechnology, 2008, 26, 417-426.