3D technology has revolutionised the entertainment industry by offering viewers the experience of being part of the action, going on in a movie rather than simply watching it. Thanks to 3D technology, we can sky walk hand in hand with George Clooney in ‘Gravity’.
The history of 3D technology can be drawn all the way back to the invention of the stereoscope by David Brewster in 1844. Last two decades have seen 3D technology replacing 2D in all walks of life, ranging from entertainment, physics, microelectronics, tissue engineering and regenerative medicine. For e.g., microelectromechanosensors (MEMS) are 3D devices produced by using soft lithography techniques. MEMS installed in air-bags in the cars have saved thousands of lives by sensing pressure levels during accidents.
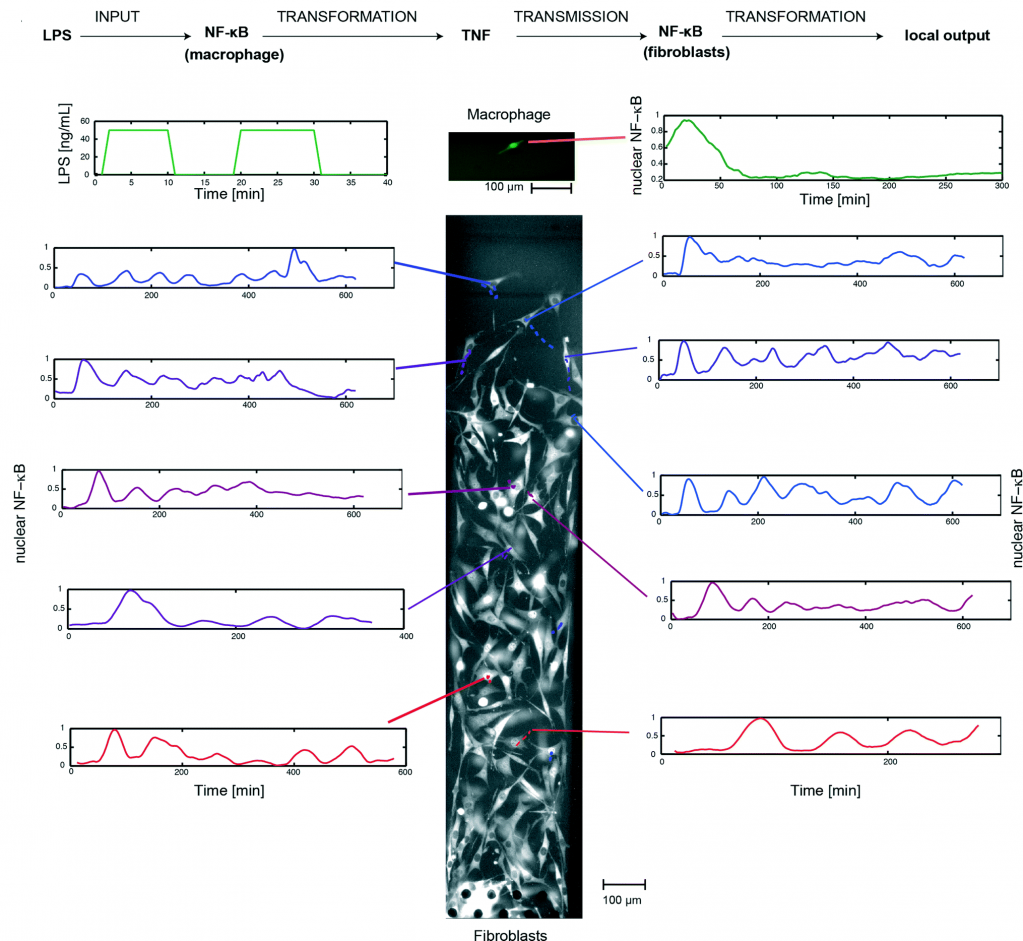
Can we use 3D technology to have a better look at the complex events happening at cellular level? One of the major challenges in tissue engineering is that the conventional approaches are mainly limited to 2D monolayers systems and do not allow manipulation of complex multilayer tissue. Cells grown on 2D substrates may respond and differentiate distinctly than those in more physiologically relevant 3D environments. The emergence of 3D technology has enabled scientists to mimic the exact cellular environments and helped to provide better insights into the cell signalling, migration and differentiation in cells.
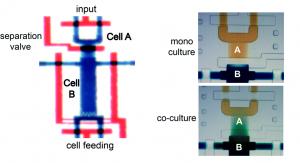
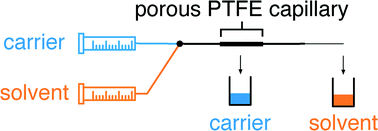
One of the ways of mimicking the cellular architectures is bio-etching which involves subtractive manufacturing. Bioetching of monolayers of cells in response to laser cuts or scratch assays is achieved by using 2D cell culture studies. But the actual biological systems such as tissues and organs are much more complex and cannot be mimicked using simple monolayers. For long time, scientists have been working on developing better technologies to address this problem. One of the ways to achieve this is 3D bio-etching.
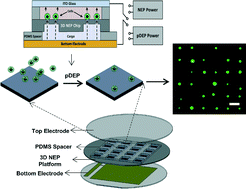
William C. Messner et al. from Tufts University in a recent article in Lab on a Chip explain the utility of 3D bioetching technique to create and shape 3D composite tissues using a microfluidics based approach. The ability to shape the 3D form of multicellular tissues and to control 3D stimulation will have a high impact on tissue engineering and regeneration applications in bioengineering and medicine as well as provide significant improvements of highly complex 3D integrated multicellular biosystems.
Can 3D bio-etching help us to design tissue architecture of our choice mimicking different biological events? Find out by reading the full paper for free* using the link below:
3D bio-etching of a complex composite-like embryonic tissue
Melis Hazar, Yong Tae Kim, Jiho Song, Philip R. LeDuc, Lance A. Davidson and William C. Messner
Lab Chip, 2015, Advance Article
DOI: 10.1039/C5LC00530B
*Access is free through a registered RSC account.