Many organisms can direct their movements according to their chemical environment. In bacteria, this chemical-directed migration (i.e. chemotaxis) is enabled by receptor-mediated signaling to the bacterial flagellar motors.1 Equipped with this remarkable sensory-motor system, bacteria can measure chemical gradients and swim towards nutritious substances (positive chemotaxis) or flee from cytotoxic chemicals (negative chemotaxis).
Bacterial chemotaxis is critical for various processes including biofilm formation, biomediation, and diseases pathogenesis.2 For example, at the onset of various digestive diseases, bacteria rely on chemotaxis to find a suitable colonization site in the human gastrointestinal tract. Thus, there is great interest in studying the rates and molecular mechanisms in these chemotactic processes.
In the past 10 years, microfluidic gradient-generators have improved the way scientists study bacterial chemotaxis;3 however, these studies have not addressed gas gradients (e.g. O2 and CO2), which are known to be important in bacterial motility.4 Microfluidics devices have enabled the generation of well-controlled chemical gradients and the measurement of bacterial response at high spatiotemporal resolution. Gradients are typically formed by flowing a sample liquid (e.g. aspartate in buffer) and a reference liquid (e.g. buffer) into the device.
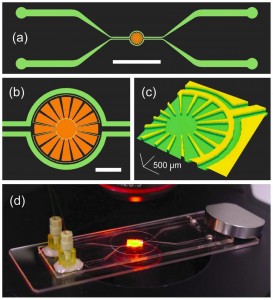
A team of researchers at the RIKEN Advanced Science Institute, Japan and Hanyang University, Korea carried out the world’s first microfluidic study of bacterial chemotaxis in gas gradients.5 The microfluidic device (see picture) comprises an isolated chamber (micro-aquarium) for bacterial culture and two bypass channels for sample and reference inputs. In this design, the sample molecules in the channel permeate through the PDMS walls (150 µm thick) and diffuse into the liquid of the micro-aquarium, facilitating the generation of a stable, shear-free gradient. The bacterial response can be monitored optically and quantified via image processing.
Using this device, the research team studied the chemotactic behaviour of Euglena gracilis microbial cells in the presence of CO2 gradients. These studies revealed that the chemotaxis of Euglena cells is rapid and is dependent on CO2 concentration. In particular, negative chemotaxis was observed at high concentrations (>15%) and positive chemotaxis was observed at low concentrations (<15%)— the maximum positive chemotaxis was observed at ~5% CO2, corresponding to the most favourable condition for photosynthesis. Interestingly, the team observed evidence of chemotactic “adaption” when they consecutively switched the sample and reference inputs.
The microfluidic device is also compatible with small molecule liquid samples such as ethanol, H2O2, and culture media. By continuously perfusing culture medium in the bypass channels, the viability of the Euglena cells in the micro-aquarium can be maintained for more than 2 months.
In summary, the research team created a microfluidic gradient-generator that is versatile and straightforward to use. The device is useful for bacterial chemotaxis studies in chemical gradients formed from gas or liquid samples. Furthermore, the device may potentially be useful for other applications including drug screening, toxicity assays, and environmental monitoring.
- H. C. Berg, Annual Review of Biochemistry, 2003, 72, 19-54.
- T. Ahmed, T. S. Shimizu and R. Stocker, Integrative Biology, 2010, 2, 604-629.
- J. Wu, X. Wu and F. Lin, Lab on a Chip, 2013, 13, 2484-2499.
- C. Douarche, A. Buguin, H. Salman and A. Libchaber, Physical Review Letters, 2009, 102, 198101.
- K. Ozasa, J. Lee, S. Song, M. Hara and M. Maeda, Gas/liquid sensing via chemotaxis of Euglena cells confined in an isolated micro-aquarium, Lab on a Chip, 2013, 13, 4033-4039