Malaysian scientists have created a flexible and environmentally friendly microfluidic device using a cloth decorating technique for printing wax onto cotton.
Dedy Wicaksono at the University of Technology Malaysia was inspired by his batik-patterned clothes to create the device. ‘Batik processing is wax patterning to create regions of differing hydrophilicity and hydrophobicity on cloth,’ says Dedy. The technique is traditionally used to prevent dye spreading from one area of cloth to another, creating coloured patterns. Using wax printing methods for paper and silk based microfluidic devices is known, but these have required specialist equipment or expensive materials.
Wicaksono’s method needs neither of these things. First, his team prepared the cotton by scouring it with sodium hydroxide and anhydrous sodium carbonate solutions. The treatment removes the outer layer, exposing underlying cellulose fibres. Both the chemical composition (increased oxygen content) and physical structure (increased surface roughness) of the fibre surface were altered, increasing the wettability and wicking rate.
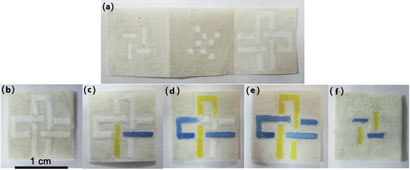
(a)-(f) are photographs showing two hydrophilic channels with different dye solutions that cross each other vertically and horizontally in three layers without mixing. (b)-(e) are front views of the device before, 5 seconds, 2 minutes and 5 minutes after dropping the dyes into different channels. (f) is the bottom layer 5 minutes after adding the dyes.
Then, they printed the pattern for the microfluidic device onto paper. The paper was dipped into hot batik wax, then dried, before the pattern was cut from the sheet and attached to the scoured cloth using a few pins. Heat treatment melted the wax again, and it spread onto the surface and into the cloth, filling the gaps in the weave and within the fibres. The fatty acids in the wax increase the hydrophobicity of the fibres where they are applied, creating barriers to liquid flow.
Dedy’s group made both 2D and 3D devices; the latter was made by folding layers of the patterned cloth. A test using ink solutions revealed the ink moving along the cloth’s hydrophilic channels, filling a device in minutes. In a further test, the team was able to detect the protein bovine serum albumin colorimetrically using the devices, with the result visible to the naked eye.
‘A key innovation here is the batik-inspired method of transferring patterned wax on paper to cotton cloth,’ comments Shashi Murthy, an expert in microfluidic devices at Northeastern University, US. He adds that the technology ‘has the potential to provide a rapid and low cost readout for analytes characterised by relatively simple colorimetric assays’.
Dedy is now investigating ways to exert more control over the liquid flow so that more complex microfluidic devices can be developed. ‘By making the channels inside a flexible cloth, we are envisioning an embeddable wearable lab in the very near future,’ he says.
Flexible microfluidic cloth-based analytical devices using a low-cost wax patterning technique
Azadeh Nilghaz, Dedy H. B. Wicaksono, Dwi Gustiono, Fadzilah Adibah Abdul Majid, Eko Supriyanto and Mohammed Rafiq Abdul Kadir
Lab Chip, 2012, Advance Article
DOI: 10.1039/C1LC20764D
Original article published at Chemistry World