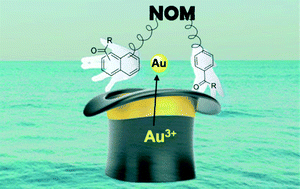
Graphical Abstract
The precious element gold can exist in various forms, the most thought of probably being the shiny solid treasured for its aesthetic qualities. However, a number of other relevant forms exist not just in the chemistry laboratory but also in nature. Gold in its common oxidation states (I and III) can be found in water sources in the environment at appreciable concentrations. The coexistence of natural organic matter (NOM) and gold dissolved in surface water opens up the possibility for photoproduction of gold nanoparticles (AuNP), a form of gold which displays toxicity towards aquatic organisms and is prone to becoming distributed through the food chain upon ingestion.
In this Environmental Science: Nano paper entitled Aqueous photoproduction of Au nanoparticles by natural organic matter: effect of NaBH4 reduction the authors’ attention is focused on investigating what NOM functionality is involved in AuNP formation, and which mechanism is being facilitated by its presence. Samples of NOM from various sources were included in the study and characterised using their content of carbonyl, quinone and aliphatic functionality. Samples with a large percentage of the former two showed an enhanced ability for AuNP production under simulated sunlight conditions, whereas a large content of the latter afforded inferior performance in this respect. In order to test whether this oxygen-containing functionality was responsibile for the facile production of AuNPs a selective chemical reducing agent, sodium borohydride (NaBH4), was chosen to remove it from the NOM samples. This modification led to Au3+ reduction by NOM being observed, albeit at a much slower rate, which confirmed the importance of the carbonyl functionality and suggested that other functionality in the NOM was ultimately responsible for performing reduction.
One way in which the carbonyl functionality could accelerate the photoreduction process is by acting as a convenient electron source following the absorption of light, with the molecular oxygen/superoxide radical pair acting as an electron shuffle between the NOM and the gold cations. Formation of the superoxide radical on irradiation of NOM solutions was followed using electron paramagnetic resonance both before and after NaBH4 treatment. Elimination of the carbonyl functionality hindered formation of the superoxide radical, however a correlation between superoxide concentration and AuNP formation rate could not be established. To test whether the superoxide radical was involved in the nanoparticle formation process, the enzyme superoxide dysmutase was added. The enzyme efficiently removed the superoxide radical, which led to an expected decrease in rate. These observations helped support the operation of the indirect reduction mechanism, however not to the dismissal of alternatives. Indeed another mechanism which is believed to operate in parallel is based on the charge transfer from the NOM directly to the gold cations in complexes formed between the two (the charge transfer mechanism).
Experiments carried out with model substrates of aromatic ketones and quinones further supported the role that had been ascribed to the carbonyl functionality. Aromatic ketones proved superior over the quinoid model compounds in the generation of nanoparticles, as faster reduction was achieved even at much lower substrate concentration and indicated that NOM quinones played only a secondary role in the production of AuNPs. Noteworthy was the decrease in photoreduction rate observed upon NaBH4 treatment of quinones, which indicated that the resulting phenolic functionality was not responsible for photoreduction as had been previously believed.
Jiahai Ma and co-authors from the School of Chemistry and Chemical Engineering have shaped our understanding for the central role that the aromatic ketone functionality in natural organic matter plays in the photoproduction of gold nanoparticles in the aquatic environment.
Read the full article for free*:
Aqueous photoproduction of Au nanoparticles by natural organi matter: effect of NaBH4 reduction
Zilu Liu, Pengfei Xie and Jiahai Ma
Environ. Sci.: Nano, 2016, Advance Article
DOI: 10.1039/C6EN00126B, Paper
—————-
 About the webwriter
About the webwriter
Dan Mercea is a PhD student in the Fuchter group at Imperial College London. He is working on developing enantioselective FLP catalysis.
—————-
*Access is free until 24/08/2016 through a registed RSC account – register here
Comments Off on Earth, wind and the sun = gold?




















 Iseult Lynch is a physical chemist specialising in understanding the interface between engineered nanomaterials and the environment (biotic and abiotic components) and how this determines their ultimate fate and behaviour.
Iseult Lynch is a physical chemist specialising in understanding the interface between engineered nanomaterials and the environment (biotic and abiotic components) and how this determines their ultimate fate and behaviour.
