A phospholipid component of plants can potentially provide versatile, environmentally benign ligands in the synthesis of asymmetric gold nanoparticles (GNPs). This paper by Benjamin Ayres (Portland State University) and Scott Reed (University of Colorado Denver), demonstrates how this can provide benefits in terms of both possible application and environmental performance of these materials.
Asymmetric GNPs have been utilised for a wide range of applications including biomedical sensors and components of electronic and photonic devices. In order to move towards a large scale production of asymmetric GNPs, a synthetic method is required that is economical and reproducible, as well as displaying suitable green credentials.
The shape and size of GNPs are crucial to governing their electronic and optical properties. There is a need therefore to gain a good understanding of the synthetic process at a molecular level, in order to optimise the process from the perspective of both desired application and environmental impact, creating a method that can be easily controlled and reproduced.
Conventional synthetic methods traditionally involve seed-mediated synthesis using alkyl ammonium salts (e.g. CTAB) as ligands. These methods are unsustainable and non-biocompatible due to the toxic nature of the compounds used, and often require excess ligand and post-synthetic processing e.g. using ligand exchange in order to be used for in vivo applications.
The method described by Ayres and Reed involves reduction of bromoauric acid by ascorbic acid in the presence of ligands derived using biogenic phospholipid extracts from crude soybean lecithin. This provides a cheap, readily available and renewable feedstock for the process, avoiding use of fossil-fuel derived materials.
A key parameter of generated GNPs is their localized surface plasmon resonance (LSPR). In order for GNPs to be suitable for medical applications (e.g. biosensors, in-vivo imaging and phototherapeutic treatments) the LSPR needs to be in the near infrared (NIR) region.
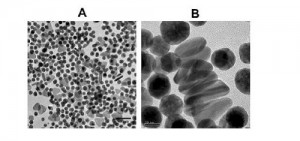
It was shown, using UV-Vis spectroscopy and high resolution transmission electron microscopy (TEM) that the described method produces a mixture of spherical and triangular prismatic GNPs that exhibited an LSPR in the NIR region as well as extended stability with no aggregation.
Furthermore, the method includes chemical identification of the specific molecular component of soy lecithin that is the source of asymmetric growth. Soy lecithin is composed predominantly (~75%) of phosphatidylchorine (PC). However, separation of different GNP shapes using preparatory gel electrophoresis, and analysis of lipid extracts by LC-MS indicated phosphatidic acid (PA) was crucial to GNP asymmetric growth.
Indeed, it was shown that GNPs produced from lower purity PC (30%) showed NIR LPSR and were stable, while higher purity PC (95%) produced only spherical GNPs which aggregated over time and displayed LSPR only in the UV-Vis spectrum. When spiked with PA, the growth solutions of PC95 displayed a second LSPR in the NIR, which shifted further red as more PA was added.
This study presents a biocompatible method for GNP production using environmentally benign ligands. It also demonstrates how gaining molecular level understanding of the synthetic process allows control over the GNP shape and size, based on lipid composition and/or specific plant material used. This therefore provides benefits both from the perspective of the desired application and the environmental impacts.
Environmental Science: Nano is providing free access upon registration* to all content published during 2014 and 2015. To get your free download of this paper, please follow the link below:
A minor lipid component of soy lecithin causes growth of triangular prismatic gold nanoparticles, Benjamin. R. Ayres and Scott. M. Reed, DOI: 10.1039/C3EN00015J
*Free access to individuals is provided through an RSC Publishing personal account. It’s quick, simple and more importantly free to register!