It’s becoming apparent to me that there are nanoparticles all over the place. My vision hasn’t increased outstandingly, but papers like this one in Environmental Science: Nano, which address materials that are used in consumer products, can bring home the relevance of this fast-paced scientific field to everyday life. Read on to find out how, if manufacturers aren’t careful, it might be possible that the suncream you use to protect your skin from UV radiation damage is introducing DNA mutations of its own.
Zinc oxide (ZnO) absorbs UVA and UVB wavelengths; exactly the ones we want to block out when we spend too long in the sun. ZnO is therefore the perfect suncream ingredient. However, micron-sized ZnO particles are bright white in visible light, and no one wants that streaky beach look. Nano-sized ZnO particles, however, are transparent to visible light and are therefore ideal for use in suncream and cosmetics that aim to protect us from UV radiation.
But there is a problem with using ZnO nanoparticles in cosmetics. They have a tendency to induce significant DNA damage and cytotoxicity.
With an increase in recent years in the use of ZnO nano structures in suncreams, efforts must be made to develop safer ZnO particles which maintain their optical properties whilst displaying reduced toxicity.
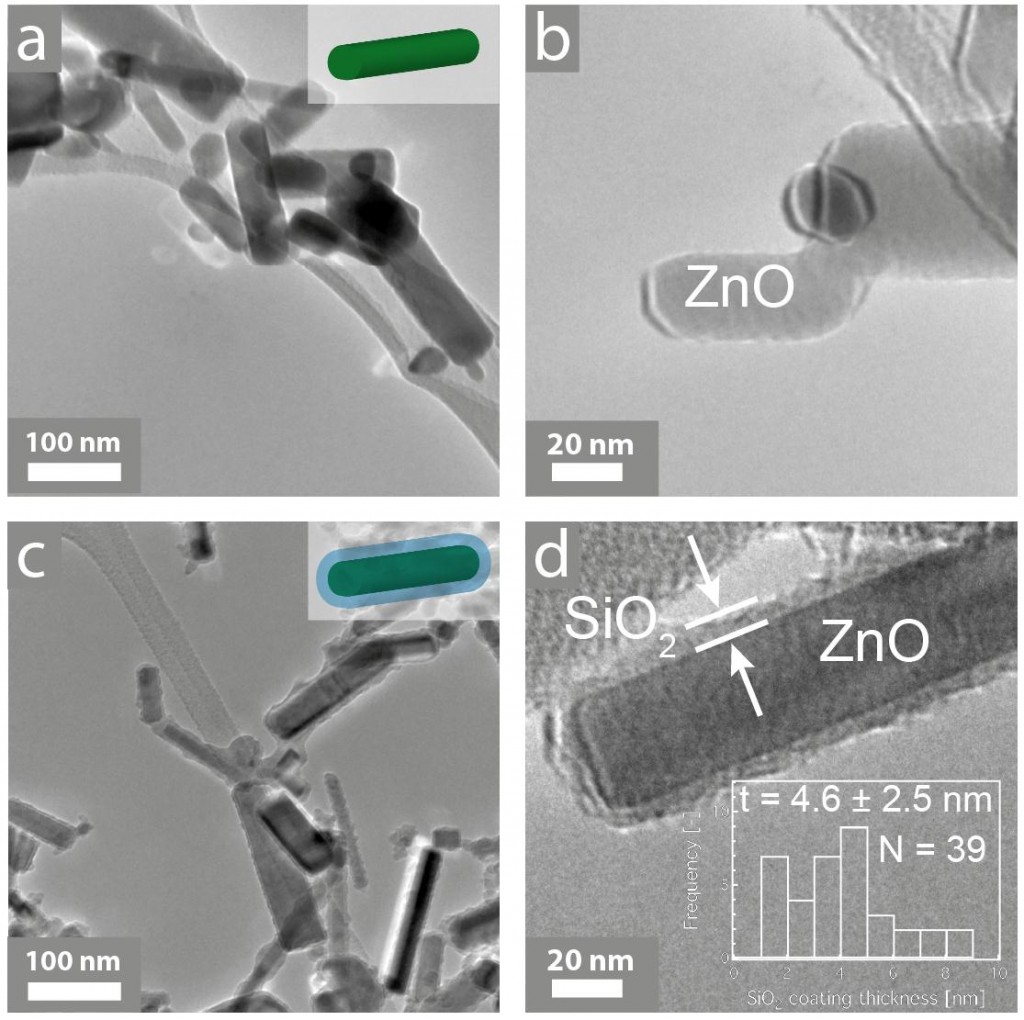
In this Environmental Science: Nano paper by Philip Demokritou and colleagues from Harvard University, ZnO nanorods were trapped in a biologically inert nanothin amorphous SiO2 coating during the gas phase of synthesis. The team then demonstrated, using human lymphoblastoid cells, that whilst encapsulation did not alter optical properties, the SiO2-coated ZnO produced significantly lower DNA damage than uncoated ZnO nanorods.
Demokritou’s method, or an extension of it, could allow manufacturers to tick all the boxes: use colourless (aesthetically pleasing) ZnO nano structures to absorb the harmful UV rays your product protects against, whilst ensuring that the wearer isn’t slapping a cytotoxin all over their skin.
Download your free* copy of this paper by following the link below:
Engineered safer-by-design, transparent, silica-coated ZnO nanorods with reduced DNA damage potential, by Philip Demokritou and colleagues, DOI: 10.1039/C3EN00062A
*Access is free through a registered RSC account – click here to register