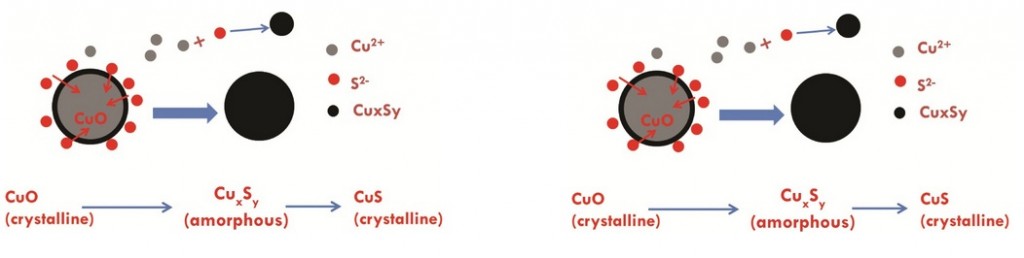
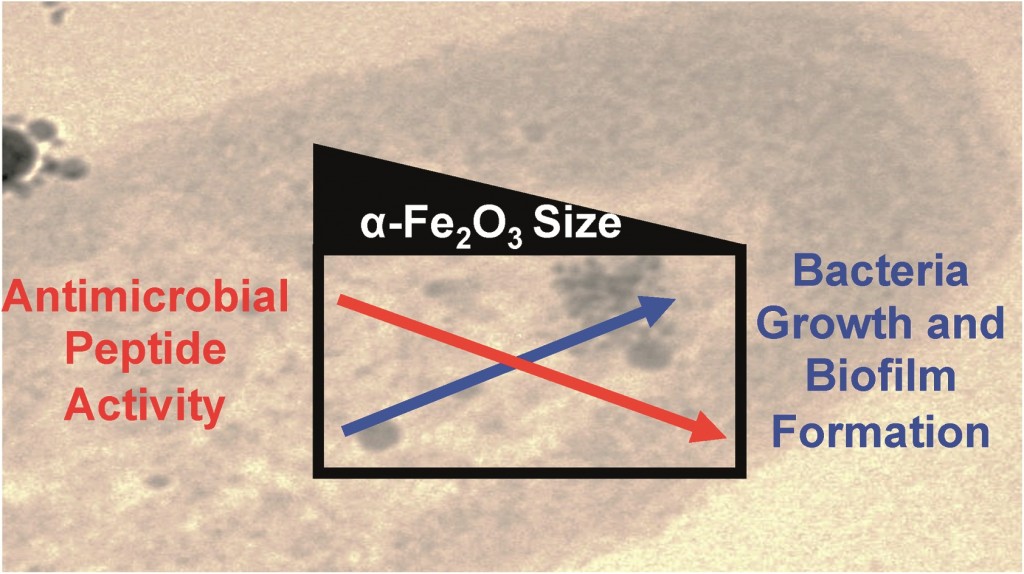
A study by Gregory Lowry and colleagues from Carnegie Mellon University suggests that Copper Oxide (CuO) nanoparticles (NPs) are sulfidized in the environment, affecting their resulting properties. Some of the affected properties, such as solubility are relevant to the toxicity of these nanoparticles in the environment.
Many nanoparticles are transformed in the environment; it is often the transformed materials which cause concern with regards to nanoparticle toxicity. There have been several papers highlighting how important it is to research the properties of nanoparticle transformations. A recent review by Nasia Von Moos provides an overview of what is currently known about environmental transformations of nanomaterials in freshwater systems, a recent paper by Julián A. Gallego-Urrea discusses the transformations which TiO2 nanoparticles undergo once they reach the aquatic environment and this research paper reports that sulfidation is an important transformation for some metal oxide nanoparticles, such as CuO NPs.
Why is sulfidation in the environment important?
Copper-based NPs are being used in semiconductors, heat transfer fluids, catalysts, batteries and many more products and technologies. Their wide spread uses will likely lead to subsequent release into the environment, raising concerns about their potential toxicity. It has already been demonstrated that CuO NPs are toxic to many organisms including crustaceans, algae and fish; although Cu2+ is more toxic to most of these organisms. It is therefore essential to determine what the products of sulfidized CuO NPs are and if they are more or less toxic to the environment when compared with pristine CuO.
Cuo NPs were characterized and sulfidized in water by inorganic sulfide. Characterization of the resulting products showed that CuO is sulfidized to several copper sulphide species including crystalline CuS (covellite), amorphous (CuxSy) species and copper sulphate hydroxide species. In previous studies it has been demonstrated shown that sulfidation decreases the solubility and metal availability of Ag and ZnO NPs. This study however shows that the sulfidation of CuO NPs breaks the trend. Sulfidation actually increased the dissolved fraction of copper compared to pristine CuO NPs. This increased release of Cu2+ and CuS nanoclusters from sulfidized NPs compared to CuO suggests that toxicity studies with pristine CuO may be misleading in environments where sulfidation is likely to occur, demonstrating that it is prudent to use environmentally transformed nanoparticles in fate, transport and toxicitiy studies rather than focusing soley on the prisitne materials. Access the full article for free* by clicking the link below.
Sulfidation of copper oxide nanoparticles and properties of resulting copper sulfide
Rui Ma, John Stegemeier, Clement Levard, James Dale, Clinton W Noack, Tittany Yang, Gordon Brown and Gregory Lowry
DOI: 10.1039/C4EN00018H
*Access is free through a registered RSC account – click here to register
The author recommends further studies which are still needed to:
- Identify the nature of the CuxSy nanoclusters.
- Assess the toxicity of sulfidized CuO NPs and CuxSy nanoclusters.
- Assess the stability of very small metal sulphide clusters (Ag, Zn and Cu) against oxidation under environmental and biological conditions.
- Assess how sulfidation of CuO NPs occurs in situ at relevant CuO/S concentration ratios and how this affects their bioavailability under realistic exposure scenarios.