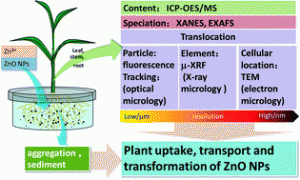
As nanoparticles find their way into more products, consumers and scientists alike are concerned about the impact their spread may have on our health. When answering this question, it is important to consider not just our direct interaction with nanoparticles through consumer products that incorporate them, but also the ways they might indirectly make their way into our environment. For instance, nanoparticles in the soil could be taken up by plants that we might later eat.
As a global food staple, maize is an ideal candidate for a comprehensive investigation of this topic. In a recent study published in Environmental Science: Nano, a team of researchers investigated the extent to which maize plants take up zinc oxide (ZnO) nanoparticles—one of the most widely used nanomaterials—and the pathways by which they do so. Their results suggest that ZnO nanoparticles dissolve into Zn2+ ions to make their way into the epidermis and roots of the plants, but rarely translocate to the shoots.
The researchers grew maize hydroponically, adding different concentrations of ZnO nanoparticles or Zn2+ ions to the water. Unsurprisingly, higher concentrations of zinc in the growth medium correlated with higher concentrations of zinc in the plants. The zinc content in the maize plants was virtually identical whether the plants were grown in ZnO solution or Zn2+ solution, suggesting that most ZnO nanoparticles make their way into maize plants by first dissolving into Zn2+, instead of being taken up whole. Zinc taken up by this pathway tended to form phosphate complexes inside the plants, largely preventing it from moving upwards into the shoots.
However, TEM imaging of plants treated with fluorescently labeled ZnO nanoparticles showed that some intact nanoparticles did find their way into the maize plants. These nanoparticles accumulated mostly in the root cortex, occasionally making their way into the vascular tissue. As with the dissolved zinc, though, the zinc oxide nanoparticles were often biotransformed to zinc phosphate and prevented from moving into the shoots.
It seems that in the case of maize, zinc oxide nanoparticles do not directly impact the parts of the plant that we would eat, but excessive accumulation of zinc compounds could potentially affect the plant’s overall health. It is unclear from this study whether the findings can be generalized to interactions between other crops and other types of nanoparticles, or even whether the pathway holds for soil-grown (as opposed to hydroponic) maize plants. Nevertheless, it provides a first step towards a comprehensive understanding of plants’ responses to and defenses against nanoparticles.
To access the full article, download a copy for free* by clicking the link below:
Accumulation, speciation and uptake pathway of ZnO nanoparticles in maize
Jitao Lv, Shuzhen Zhang, Lei Luo, Jing Zhang, Ke Yang and Peter Christie
DOI: 10.1039/C4EN00064A
Liked this blog post? Read Laurel’s previous entry on how rare earth elements trace nanoparticles through the environment.
* Access is free through a registered RSC account – click here to register