Evaluating the biosafety and environmental fate of carbon nanomaterials can be aided by the use of 13C isotopic labelling to improve their in vivo quantification and monitoring. This study conducted at the Chinese Academy of Sciences, Beijing discusses this approach.
The widespread use of carbon nanomaterials (NMs) such as fullerenes in a diverse range of applications such as biomedicine, electronics, and industrial practices will inevitably lead to their interaction with environmental and biological systems. The major barrier to the large-scale production and use of carbon NMs is fully understanding their biosafety and assessing how their unique structure and properties influence their potential effects.
To address this issue will require better knowledge of the exposure, pharmacokinetics, biodistribution and toxicity of carbon NMs. There is currently a lack of simple quantification methods for in vivo carbon NMs. In the past quantification of carbon NMs in blood and body tissue has been achieved by methods based on high performance liquid chromatography (HPLC) coupled with mass spectrometry (MS). However, other reliable methods are also available.
Isotopic labelling is also a favourable option. This commonly involves use of radioactive isotopes e.g. 14C, and 125I. However, these are subject to a number of drawbacks, as the synthesis and detection of these radioactive isotopes are complicated and this also leads to the creation radioactive wastes. Additionally, most radioactive isotopes can only be used for studying functionalized fullerenes.
The use of stable isotopes for fullerene labelling may avoid these problems. This approach of coupling the use of stable isotope labels with isotope ratio mass spectrometry (IRMS) detection has been used previously to quantify other carbon NMs such as quantum dots and nanotubes. This study by Xue-Ling Chang and co-workers represents the first time this approach of using 13C stable isotopic labelling on the carbon skeleton of fullerene has been attempted.
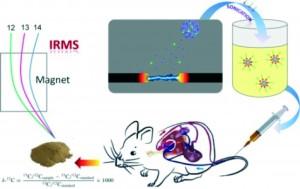
In this study, 13C-enriched fullerene (C60) was synthesised by the arc discharge method and purified by HPLC. The labelled fullerenes were dispersed in aqueous solutions assisted by CS2 and administered to test mice via i.v injection. Blood, tissue and organ samples were then collected and 13C enriched C60 content were monitored and quantified using IRMS.
The enrichment of the 13C on the C60 skeleton was confirmed by assessing the MS and IR spectra data. The pharmacokinetics of the labelled fullerenes was investigated using both one-compartmental and two compartmental models. C60 was shown to very rapidly clear from blood stream with a blood circulation half-life of 14 minutes calculated. The labelled C60 displayed selective accumulation in the RES organs, particularly, the liver, spleen and lungs with slight decreases noted within 24 hours of exposure.
The study demonstrates the feasibility of using 13C stable isotopically labelled fullerenes to trace and quantitatively monitor the bio-behaviour of carbon NMs like fullerenes in vivo. Researchers can therefore better trace the absorption, distribution, metabolism, and excretion of these materials. This approach is preferable as it does not introduce foreign atoms onto, nor does it damage or disrupt, the carbon network. The fullerene therefore retains its intrinsic structure so the labels will reflect the real properties of C60 structures.
This work provides a potential new platform to study the environmental and biological fate of carbon NMs, offering a sensitive, reliable and non-destructive method trace their effects in vivo. The authors also identify further areas of research for which the approach can be used, including the effects of long-term exposure, consequences of surface functionalisation, and potential metabolism mechanisms of carbon NMs.
Read the full paper by following the link below. Your copy is free with registration to an RSC account
Quantification of carbon nanomaterials in vivo: direct stable isotope labeling on the skeleton of fullerene C60, by Xue-Ling Chang et al. DOI: 10.1039/c3en00046j