Titanium Oxide nanoparticles (TiO2 NPs) – given their wide applications, exposure scenarios and classification as a class 2B carcinogen (International Agency of Research on Cancer), it is high time for the precise and accurate quantitative analysis of TiO2 NP contamination in environmental samples. Being one of the metal oxides that is extremely hard to solubilize makes accurate and precise measurement of TiO2 a challenge. Recent investigations using mixed acid digestion and alkali potassium hydroxide (KOH) fusion has given improved recoveries of TiO2 when compared to the conventional methods. This is great news for all of us who struggle to quantify TiO2 nanoparticles in complex environmental matrices! However, as with all nanoscale materials there are complications arising from size and polymorph dependent thermodynamic stabilities as well as chemical reactions between Ti and other sample matrices, especially at elevated temperature and pressure. Thus, R. G. Silva and coworkers of United States Environmental Protection Agency (US-EPA) investigate the digestibility of different polymorphs of TiO2 NPs; anatase, rutile and brookite. These samples were used for spiking environmental matrices consisting of river sediment and clay minerals (bentonite and kaolinite). Furthermore, a portion of these were subjected to heat (300OC) and pressure (10.3 bar) treatment to investigate its impact on the Ti recovery from the TiO2 NPs.
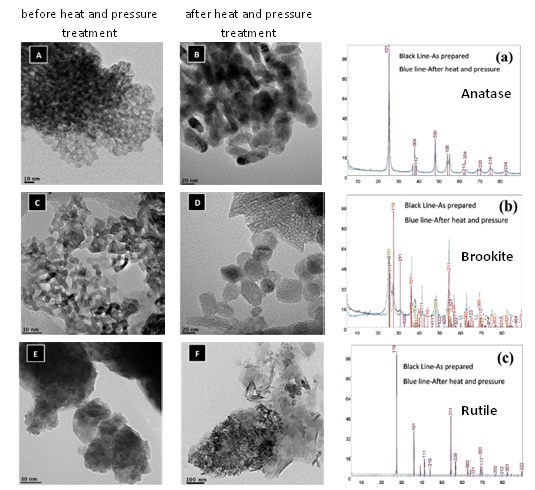
Extensive characterization of all three nanoparticle samples with respect to size, shape, crystallinity and surface area before and after the heat and pressure treatments showed significant changes in the physicochemical properties of anatase and brookite. Rutile on the other hand was resistant to changes. In terms of digestion, acid digestion resulted in relatively lower Ti concentration for the pure TiO2 NP samples that underwent heat and pressure treatment. In contrast, alkali fusion resulted in increased levels of Ti. Nevertheless, when the TiO2 NP polymorphs were blended in the environmental matrices, for anatase and brookite the recoveries were similar for both types of digestions. However, for the recovery of rutile the alkali fusion method proved to be superior to that of the mixed acid method. Therefore, this work recommends using the alkali fusion method for the extraction of Ti from TiO2 NP contaminated unknown environmental samples.
To access the full article, download a copy for free* by clicking the link below.
Polymorph-dependent titanium dioxide nanoparticle dissolution in acidic and alkali digestions
R. G. Silva, M. N. Nadagouda, C. L. Patterson, Srinivas Panguluri, T. P. Luxton, E. Sahle-Demessieb and C. A. Impellitterib
DOI: 10.1039/C3EN00103B
*Access is free through a registered RSC account – click here to register