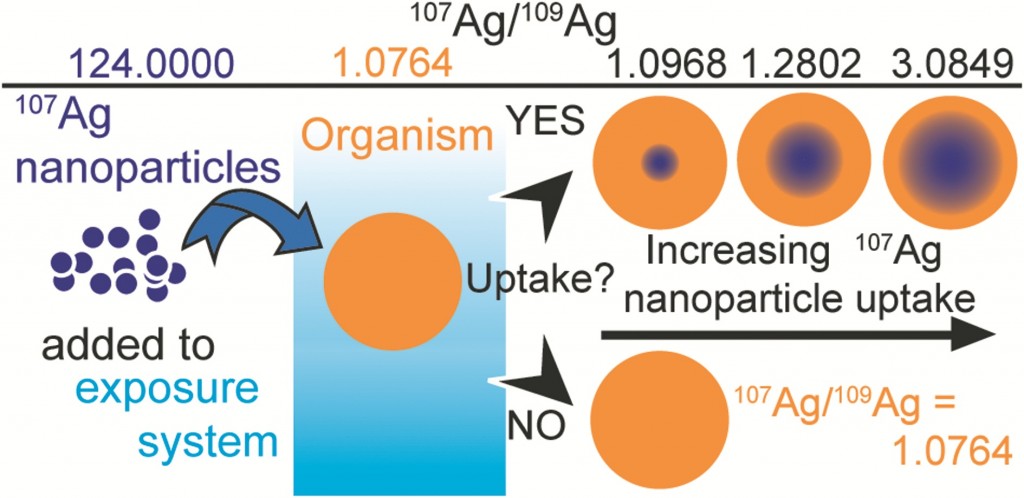
To understand the fate of silver nanoparticles (AgNPs) in the environment they need to be traced in complex natural samples. Often, studies that interrogate the environmental fate of AgNPs require AgNP concentrations that exceed realistic environmental levels. Potentially, this produces unreliable results, as high particle concentration could induce and identify distinct patterns of particle behaviour that is not relevant for ‘realistic’ environmental concentrations. Adam Laycock, from Imperial College London, and colleagues investigate whether the same effects are also observed at low, environmentally relevant AgNP concentrations.
Stable isotope labelling is an attractive method of labelling which relies on the detection of changes when a contaminant is introduced to a system. Previous methods of tracing nanomaterials have had significant drawbacks. For example, fluorescent coatings can affect the surface chemistry of the nanomaterials causing dissociation. Radiolabelling, another technique used, involves the use of specialist equipment and licenses are required for the handling of radioactive material. Recent studies have shown that stable isotope tracing can be applied to nanomaterials – but first, the labelled NPs must be specifically prepared from a single, highly enriched stable isotope form of the elements.
In this study existing protocols were examined to develop techniques for the optimized preparation of isotopically labelled AgNPs. Three protocols were applied to produce particles with a variety of target sizes, with enriched 107Ag and natural Ag. The results show that the methods are suitable for small scale synthesis of stable labeled AgNPs at yields of approximately 80%. The labelling process does not generate unusual particle properties, demonstrating that isotopically modified AgNPs are equivalent to AgNPs with a natural isotope composition.
The authors finalise their study by presenting a series of calculations which reveal that stable isotope labelling can increase the detection sensitivity of AgNPS by at least a factor of 40, and possibly by up to 4000x in comparison to commonly employed bulk Ag concentration measurements. This approach of tracing nanomaterials is highly versatile, the label cannot be lost by dissociation or degradation, the element remains traceable and the use of enriched stable isotopes provides an extremely selective and sensitive means of elemental tracing, even in the presence of high natural background levels.
To read the full experiment for free*, download the paper now:
Synthesis and characterization of isotopically labeled silver nanoparticles for tracing studies
Adam Laycock, Björn Stolpe, Isabella Römer, Agnieszka Dybowska, Eugenia (Éva) Valsami-Jones, Jamie R Lead and Mark Rehkamper
DOI: 10.1039/C3EN00100H
*Access is free through a registered RSC account – click here to register