The adventitious protein adsorption on to nanoparticles (NPs), commonly known as the NP-protein corona is in the limelight for many good reasons including its influence on the NP uptake, accumulation and ultimate cellular fate. The consequences of this phenomenon can work in different ways. NP-protein corona can lower the toxicity of NPs when compared to the “bare” NPs but at the same time facilitate crossing biological barriers that can trigger activation of specific regulatory pathways. The particle size, composition and surface properties of NPs are known to influence the composition of the NP-protein corona. Thus, Korin Wheeler, from Santa Clara University, and coworkers investigate the protein populations across the NP-protein corona using silver nanoparticles (AgNPs) in the presence of Yeast (Saccharomyces cerevisiae) proteins (YPE) with the aid of mass spectrometry (MS) proteomics.”
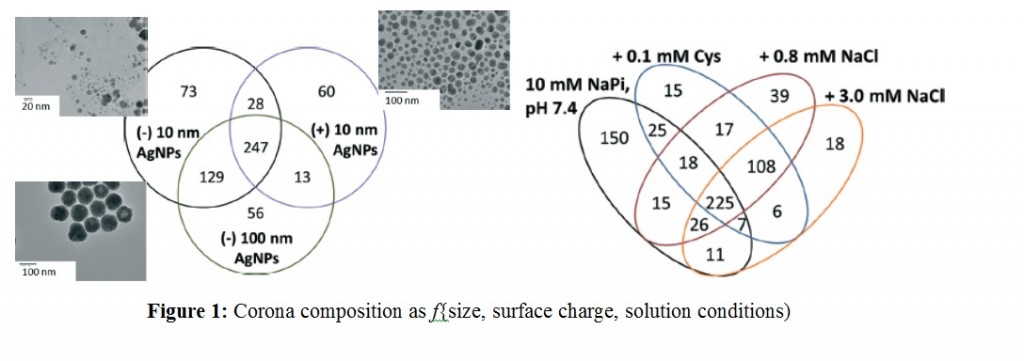
Experiments were designed to probe the effects of AgNP size, surface charge and solution conditions. Two different sizes of AgNPs (10 nm vs 100 nm) with anionic (citrate) and cationic (branched polyethyleneimmine – BPEI) coatings were selected. Protein corona formation was conducted under 0.8 mM NaCl, modeled after freshwater salinity; 3.0 mM NaCl, mimicking mitochondrial salinity; and 0.1 mM Cys, which was shown to mediate NP toxicity. After incubating AgNPs with the YPE, bound and unbound proteins were separated via centrifugation, digested with trypsin followed by LC-MS/MS analysis.
Over 500 different proteins were identified as bound and unbound but no trends were reported linking the molecular weight, pI, length, or the amino acid composition of proteins to their enrichment in the corona. However, AgNP surface charge played a stronger role with those having similar coatings sharing majority of corona population. This highlighted electrostatic modulation of protein affinity for AgNPs under low ionic strength conditions. Further studies with NaCl and cys to simulate more environmentally and biologically relevant conditions revealed more changes in the protein corona populations and modifications in their solution phase behavior in terms of aggregation and sedimentation. However, under all the variable factors there is a population of ubiquitous proteins that get enriched in the corona as shown in the Figure below.
The findings of this research is expected to contribute towards better understanding the bio physicochemical factors mediating NP-corona formation, their characterization and the development of predictive models within the environment.
To access the full article, download a copy for free* by clicking the link below.
Silver nanoparticle protein corona composition compared across engineered particle properties and environmentally relevant reaction conditions
Richard Eigenheer, Erick R. Castellanos, Meagan Y. Nakamoto, Kyle T. Gerner, Alyssa M. Lampe and Korin E. Wheeler
DOI: 10.1039/C4EN00002A
*Access is free through a registered RSC account – click here to register