With the level of engineered nanomaterials (EMNs) in the environment continuously increasing, there are rising concerns with regards to their potential environmental impact. In recent years a more accurate understanding of particle behaviour in complex systems has been gained. Numerous studies have investigated the environmental hazards of ENMs, but the link between these two aspects is less developed. There are still considerable knowledge gaps with respect to ENMs bioavailability in the environment.
A review by Nadia Von Moos, from the University of Geneva, and colleagues provides an overview of what is currently known about environmental transformations of nanomaterials as well as their interactions with, and their toxicity towards bacteria and microalgae.
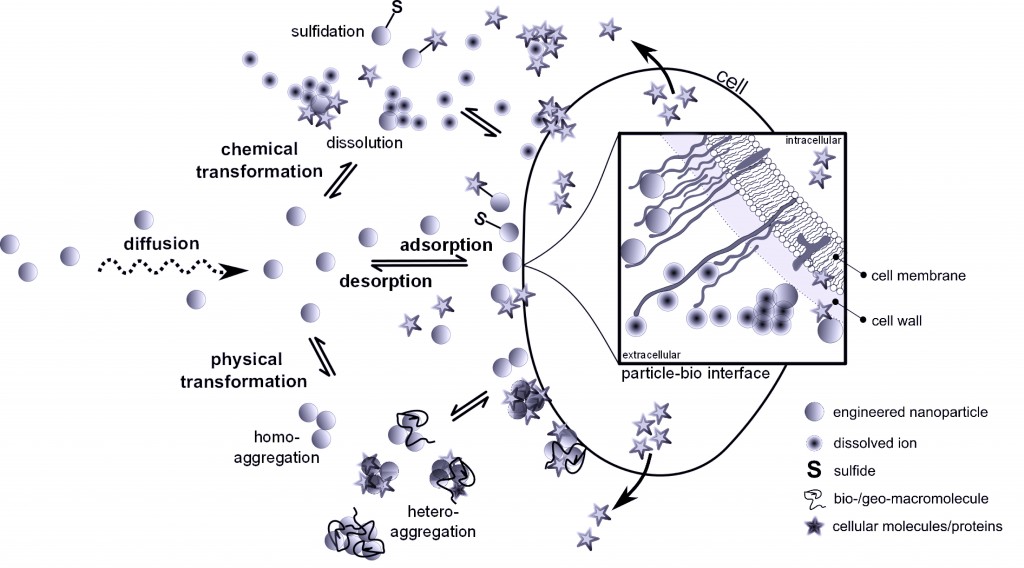
This diagram shows the processes at the medium – bio-interface underlying the bioavailability of ENMs to aquatic microorganisms (AMO). The bioavailability is dependent on many processes, such as chemical and physical transformations, adsorption and desorption, internalization, intracellular fate, agglomeration, dissolution and surface transformations. The importance of ENMs’ material characteristics has been reviewed before, but this review emphasises the environmental factors affecting the above processes. A quantitative understanding of ENM bioavailability requires insights into their behaviour during transport from the ambient medium to the AMO interface and of the processes underlying adsorption, internalization as well as intracellular fate – all of which are discussed in this review.
This review clearly concludes that there are still considerable knowledge gaps with respect to the effects of agglomeration on bioavailability, exact uptake routes, intracellular compartmentalization as well as dissolved organic matter-protein competition on the surface of internalized engineered nanoparticles. This review can be used to guide future research efforts in nanomaterial hazard and risk assessment. To read more, download your free* copy by clicking the link below:
Nadia Von Moos, Paul Bowen and Vera I Slaveykova
DOI: 10.1039/C3EN00054K
*Access is free through a registered RSC account – click here to register