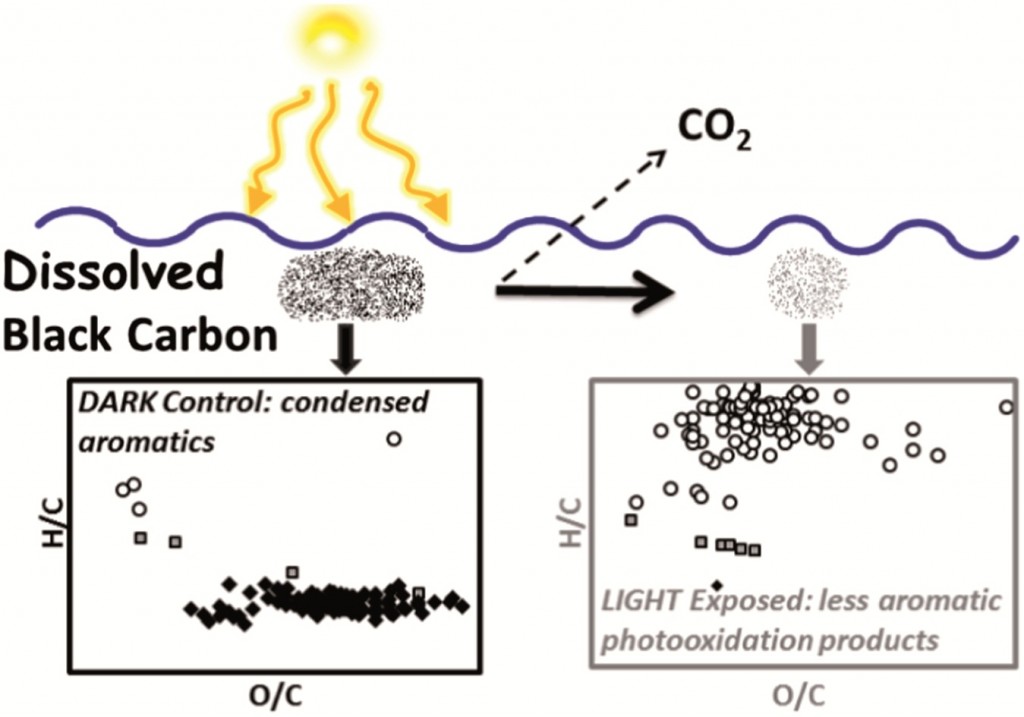
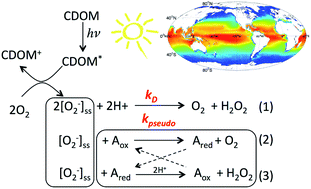
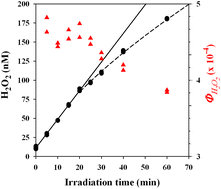
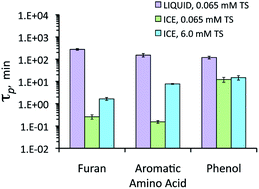
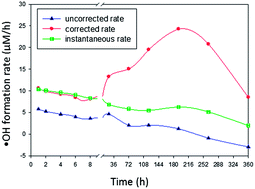
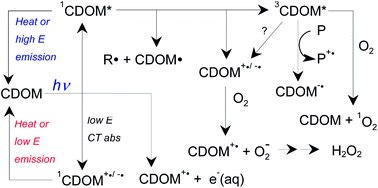
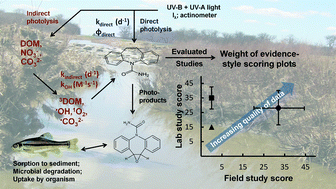
The fouth of our Introducing series of blog posts features Editorial Board member Professor Yngvar Thomassen – we’re very pleased to welcome him to the board and post his profile and research vision:
Yngvar is currently a Research Director for the Department of Chemical and Biological Work Environment at the National Institute of Occupational Health in Oslo – where he has spent 35 years of his professional life. After graduating from the Department of Analytical Chemistry at the University of Oslo in 1973, Yngvar spent a year at the Norwegian Defence Institute before taking a post research associate position, back at the University of Oslo.In 1978 he worked for the Department of Environmental Studies and Geology at the University of Toronto as a visiting scientist. He has since been appointed as a Professor in Environmental chemistry, Department of Plant and Environmental Science, at the Norwegian University of Life Sciences.
MY RESEARCH VISION:
My passion for research and teaching derives from my quest for social and environmental interest. This has inspired me throughout my professional life as an analytical chemist. From occupational and personal use of products to nutritional intake people are exposed to a variety of chemical agents – many essential or non-essential compounds with the potential to affect our health. Analytical science has been and is an important instrument in chemical exposure science which strives to collect and analyse qualitative and quantitative information which is needed to understand the nature of contact between people and chemical stressors. There are a continuous demand for exposure science information to meet the need to understand the fate of stressors and to establish exposure data, not only for the existing chemical agents, but also for the thousands of new chemicals introduced into the marked each year.
Although analytical science has brought about a recent revolution in exposure characterization and dose assessment, now even able to reach the nanoscopic domain and fundamental limits of atom or molecule detection, these developments need to be further integrated into more portable and direct reading instruments for biological and environmental monitoring for faster identification of chemical stressors affecting our health. Of special importance is further improvement of ambient, indoor and work-room air qualities since airborne contaminants still seriously affects the health of workers and the global population at large. In order to achieve this, an expanded integrated vision in exposure science which consider exposures from source to dose, over time and space, as well as multiple stressors are required. Thus, the society should give priority to
a) educate the next generation of analytical and exposure scientists
b) further develop new and improve existing instrumentation
c) stimulate to strategic collaboration across scientific boarders
d) develop prevention and intervention strategies to reduce any related health problems
e) improve quality of exposure data collected and make them available to help set priorities and inform policy