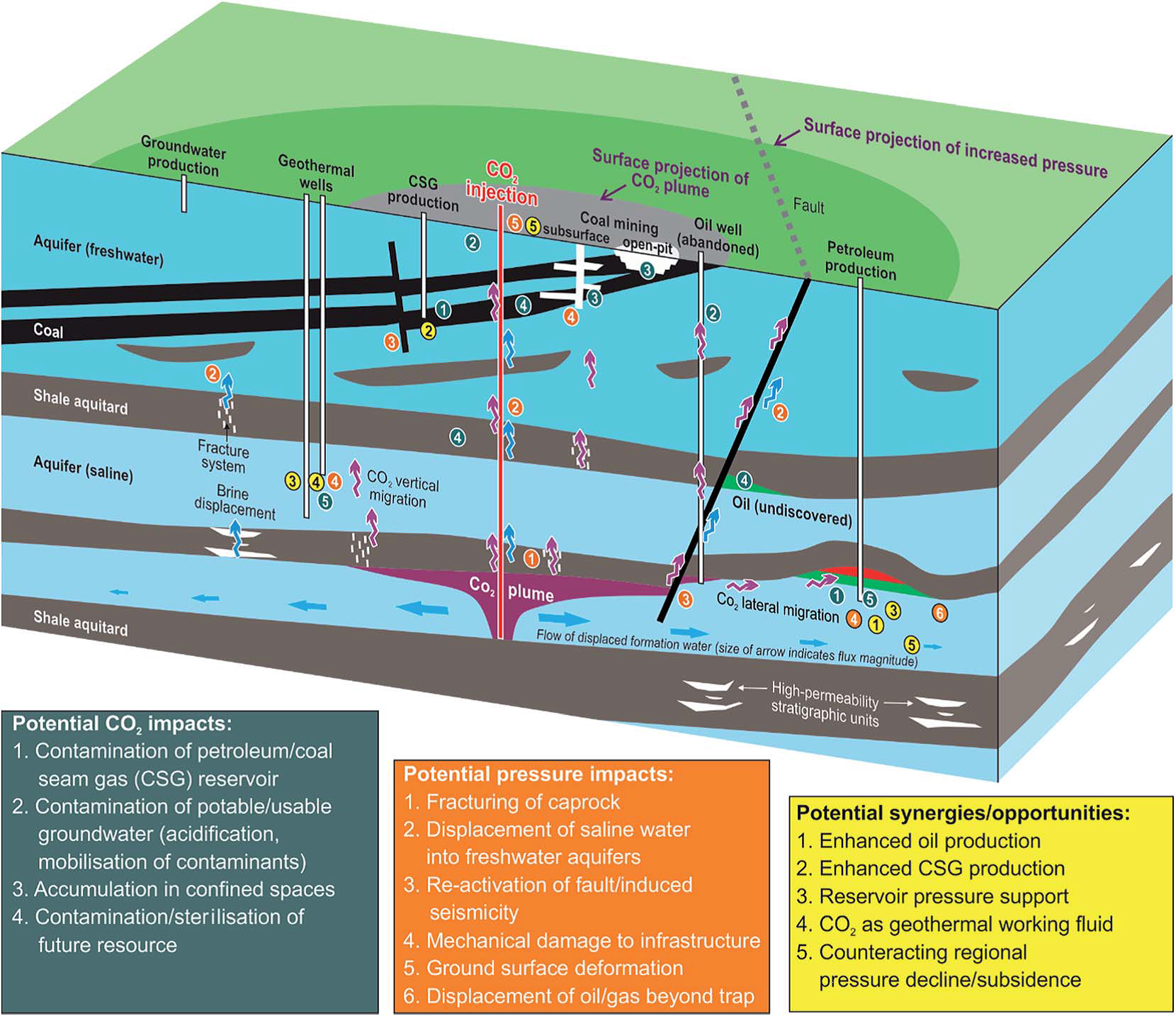
In the continuing drive to meet the world’s future energy needs, few can have missed the increased recent prominence of unconventional hydrocarbons. The extraction of resources such as shale gas, using techniques like hydraulic fracturing (or fracking) has gained attention from around the world, both for its potential to fulfil our energy requirements, as well as due to concerns around possible environmental impacts.
Chemistry has a strong role to play in helping us to understand potential environmental impacts, particularly areas such as air and water quality. The Royal Society of Chemistry supports the research community examining these issues, as chemical sciences research can help to inform broader policy decisions on this issue. We publish papers and books across our portfolio of journals, including those that encourage scientific debate and have actively supported those in the research community to share knowledge and advance science. To mark the publication of a report that explores research questions in this area, we have gathered some of our best publications on this topic to share with readers.
Unconventional hydrocarbon extraction in different countries has evolved at different rates. In the US there has been rapid exploration that has led to commercial scale production, whilst in the UK we are at a much earlier stage of the debate. Examining the situation in places like the US, which has a comparatively more established shale gas industry, can help us to determine what is known about the potential environmental impacts and where research, including chemistry, can help to address unknowns.
In November 2015, the UK Natural Environment Research Council (NERC), the US National Science Foundation (NSF) and the Environment, Sustainability and Energy Division of the Royal Society of Chemistry brought together researchers from the US and the UK at a workshop on Improving the Understanding of the Potential Environmental Impacts Associated with Unconventional Hydrocarbons. The aim of the workshop was to share knowledge in this rapidly changing area, particularly with respect to identifying research gaps and areas where future research may be needed. The range of topics covered by the workshop was broad, including areas such as air quality and wastewater treatment, which have a direct link with the chemical sciences, through to seismicity and socioeconomic impacts.
Participants at the workshop had the opportunity to present current research in their field, taking into account the specific situation in their country. Discussions at the workshop examined similarities and differences between the different nations, leading onto the identification of knowledge gaps and future research needs.
The workshop’s co-chairs, Professor Richard Davies of Newcastle University and Professor Danny Reible at Texas Tech used the discussions from the meeting to produce a report, Joint US-UK Workshop on Improving the Understanding of the Potential Environmental Impacts Associated with Unconventional Hydrocarbons. The report captures key research gaps and needs in a whole range of areas from community engagement, to human health to waste water management.
Professor Richard Davies commented: “This workshop represented a valuable opportunity to prioritise and tailor research questions that could help us to better understand any potential environmental impacts if unconventional hydrocarbon extraction were to take place in the UK. The report examines both near-term and long-term research priorities for the research communities working in this area”.
The report will be relevant to researchers working on unconventional hydrocarbon extraction, outlining future research opportunities and needs. You can watch Professor Fred Worrall, one of the UK workshop participants talk about some of the points covered at the workshop in his lecture Exploring the impact of the unknown: a potential UK shale gas industry. Fred’s work on monitoring emissions relating to onshore oil and gas operations is also the subject of a recent Education in Chemistry article.
We hope that you will enjoy reading about both recent research advances and future areas for investigation in an area that will likely continue to feature in both scientific and public discourse.
Books
Fracking
Editors: R E Hester, R M Harrison
Print publication date: 02 Sep 2014
DOI: 10.1039/9781782620556
Principles and Practice of Analytical Techniques in Geosciences
Editor: Kliti Grice
Print publication date: 11 Sep 2014
DOI: 10.1039/9781782625025
Reviews
Evolving shale gas management: water resource risks, impacts, and lessons learned
Brian G. Rahm and Susan J. Riha
Environ. Sci.: Processes Impacts, 2014,16, 1400-1412
DOI: 10.1039/C4EM00018H
Use of stable isotopes to identify sources of methane in Appalachian Basin shallow groundwaters: a review
J. Alexandra Hakala
Environ. Sci.: Processes Impacts, 2014,16, 2080-2086
DOI: 10.1039/C4EM00140K
Unconventional oil and gas extraction and animal health
M. Bamberger and R. E. Oswald
Environ. Sci.: Processes Impacts, 2014,16, 1860-1865
DOI: 10.1039/C4EM00150H
Practical measures for reducing the risk of environmental contamination in shale energy production
Paul Ziemkiewicz, John D. Quaranta and Michael McCawley
Environ. Sci.: Processes Impacts, 2014,16, 1692-1699
DOI: 10.1039/C3EM00510K
 Air quality concerns of unconventional oil and natural gas production
Air quality concerns of unconventional oil and natural gas production
R. A. Field, J. Soltis and S. Murphy
Environ. Sci.: Processes Impacts, 2014,16, 954-969
DOI: 10.1039/C4EM00081A
Analysis
Deciphering the true life cycle environmental impacts and costs of the mega-scale shale gas-to-olefins projects in the United States
Chang He and Fengqi You
Energy Environ. Sci., 2016,9, 820-840 DOI: 10.1039/C5EE02365C
Wells to wheels: water consumption for transportation fuels in the United States
David J. Lampert, Hao Cai and Amgad Elgowainy
Energy Environ. Sci., 2016,9, 787-802
DOI: 10.1039/C5EE03254G
Papers
Solid-phase extraction followed by gas chromatography-mass spectrometry for the quantitative analysis of semi-volatile hydrocarbons in hydraulic fracturing wastewaters
Julia Regnery, Bryan D. Coday, Stephanie M. Riley and Tzahi Y. Cath
Anal. Methods, 2016,8, 2058-2068
DOI: 10.1039/C6AY00169F
Partitioning of naturally-occurring radionuclides (NORM) in Marcellus Shale produced fluids influenced by chemical matrix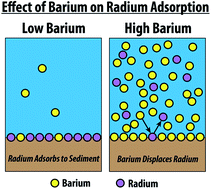
Andrew W. Nelson, Adam J. Johns, Eric S. Eitrheim, Andrew W. Knight, Madeline Basile, E. Arthur Bettis III, Michael. K. Schultz and Tori Z. Forbes
Environ. Sci.: Processes Impacts, 2016,18, 456-463
DOI: 10.1039/C5EM00540J
A liter-scale microbial capacitive deionization system for the treatment of shale gas wastewater
Casey Forrestal, Alexander Haeger, Louis Dankovich IV, Tzahi Y. Cath and Zhiyong Jason Ren
Environ. Sci.: Water Res. Technol., 2016,2, 353-361
DOI: 10.1039/C5EW00211G
Detection of water contamination from hydraulic fracturing wastewater: a μPAD for bromide analysis in natural waters
Leslie J. Loh,a Gayan C. Bandara,a Genevieve L. Webera and Vincent T. Remcho*a
Analyst, 2015,140, 5501-5507
DOI: 10.1039/C5AN00807G
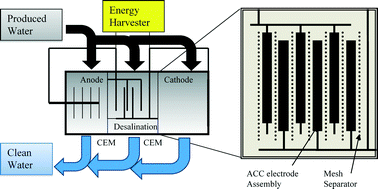 Microbial capacitive desalination for integrated organic matter and salt removal and energy production from unconventional natural gas produced water
Microbial capacitive desalination for integrated organic matter and salt removal and energy production from unconventional natural gas produced water
Casey Forrestal, Zachary Stoll, Pei Xu and Zhiyong Jason Ren
Environ. Sci.: Water Res. Technol., 2015,1, 47-55
DOI: 10.1039/C4EW00050A
Stimuli-responsive/rheoreversible hydraulic fracturing fluids as a greener alternative to support geothermal and fossil energy production
H. B. Jung, K. C. Carroll, S. Kabilan, D. J. Heldebrant, D. Hoyt, L. Zhong, T. Varga, S. Stephens, L. Adams, A. Bonneville, A. Kuprat and C. A. Fernandez
Green Chem., 2015,17, 2799-2812
DOI: 10.1039/C4GC01917B
Geo-material microfluidics at reservoir conditions for subsurface energy resource applications
Mark L. Porter, Joaquín Jiménez-Martínez, Ricardo Martinez, Quinn McCulloch, J. William Carey and Hari S. Viswanathan
Lab Chip, 2015,15, 4044-4053
DOI: 10.1039/C5LC00704F
Shale gas-to-syngas chemical looping process for stable shale gas conversion to high purity syngas with a H2:CO ratio of 2:1
Siwei Luo, Liang Zeng, Dikai Xu, Mandar Kathe, Elena Chung, Niranjani Deshpande, Lang Qin, Ankita Majumder, Tien-Lin Hsieh, Andrew Tong, Zhenchao Sun and Liang-Shih Fan
Energy Environ. Sci., 2014,7, 4104-4117
DOI: 10.1039/C4EE02892A
Organic compounds in produced waters from shale gas wells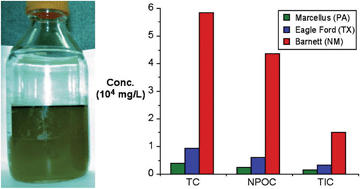
Samuel J. Maguire-Boyle and Andrew R. Barron
Environ. Sci.: Processes Impacts, 2014,16, 2237-2248
DOI: 10.1039/C4EM00376D
Automated method for determining the flow of surface functionalized nanoparticles through a hydraulically fractured mineral formation using plasmonic silver nanoparticles
Samuel J. Maguire-Boyle, David J. Garner, Jessica E. Heimann, Lucy Gao, Alvin W. Orbaek and Andrew R. Barron
Environ. Sci.: Processes Impacts, 2014,16, 220-231
DOI: 10.1039/C3EM00718A
Comments Off on Unconventional hydrocarbons – Understanding the Potential Environmental Impacts














 Air quality concerns of unconventional oil and natural gas production
Air quality concerns of unconventional oil and natural gas production
 Microbial capacitive desalination for integrated organic matter and salt removal and energy production from unconventional natural gas produced water
Microbial capacitive desalination for integrated organic matter and salt removal and energy production from unconventional natural gas produced water







 We are pleased to announce that
We are pleased to announce that