Degradation by sunlight is an important sink for black carbon in surface waters. This study by researchers at University of Michigan and Old Dominion University in Norfolk, Virginia demonstrates how partial degradation of condensed aromatic compounds is the primary mechanism for this process.
The predicted frequency of wildfire activity during the 21st century will lead to increased production of black carbon (BC) in the environment. The recalcitrant nature of BC means it displays a relatively long residence time in soils, thus constituting a key sink for atmospheric CO2.
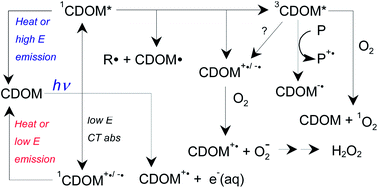
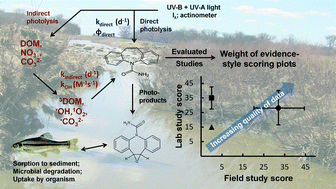
Abiotic and microbial degradation of condensed aromatics of BC in soils can lead to transfer of water-soluble BC components to surface waters, as well as particulate BC. This will expose both particulate and dissolved BC components to degradation by sunlight. The condensed aromatic component of BC in surface waters can be photo-oxidized either completely, forming CO2 or partially, forming compounds no longer be detected as BC.
Identifying the contribution of complete and partial BC photodegradation is key to understanding the extent and speed to which CO2 is returned to the carbon cycle. To date no study has investigated the relative importance of these two competing reaction pathways in surface waters. This study by Collin Ward and co-workers represents the first quantitative assessment of partial vs. complete photoxidation of BC in dissolved and particulate phases.
Previously, degradation of condensed aromatics has investigated using FT-ICR-MS or detection of molecular markers such as benzene polycarboxylic acids (BPCAs). However this approach cannot distinguish between complete and partial oxidation.
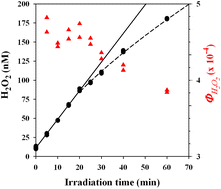
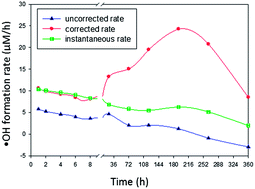
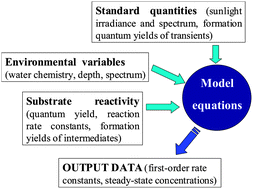
In this study, aqueous suspensions of particulate BC (BC-p) and solutions of dissolved BC (BC-d) were prepared from charring of arctic biomass, mimicking BC produced from wildfires. These were exposed to natural sunlight in a 17 hour exposure experiment. Complete and partial oxidation were quantified by monitoring CO2 production and O2 consumption using a dissolved inorganic carbon (DIC) analyser and membrane inlet mass spectrometer (MIMS) respectively.
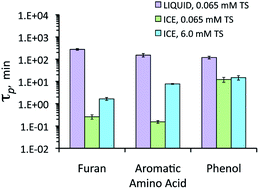
Complementing this approach, the study also investigated the shift in chemical/optical properties of BC during exposure using UV-Vis absorbance, fluorescence spectroscopy and FT-ICR-MS. Results were assessed relative to dark control tests.
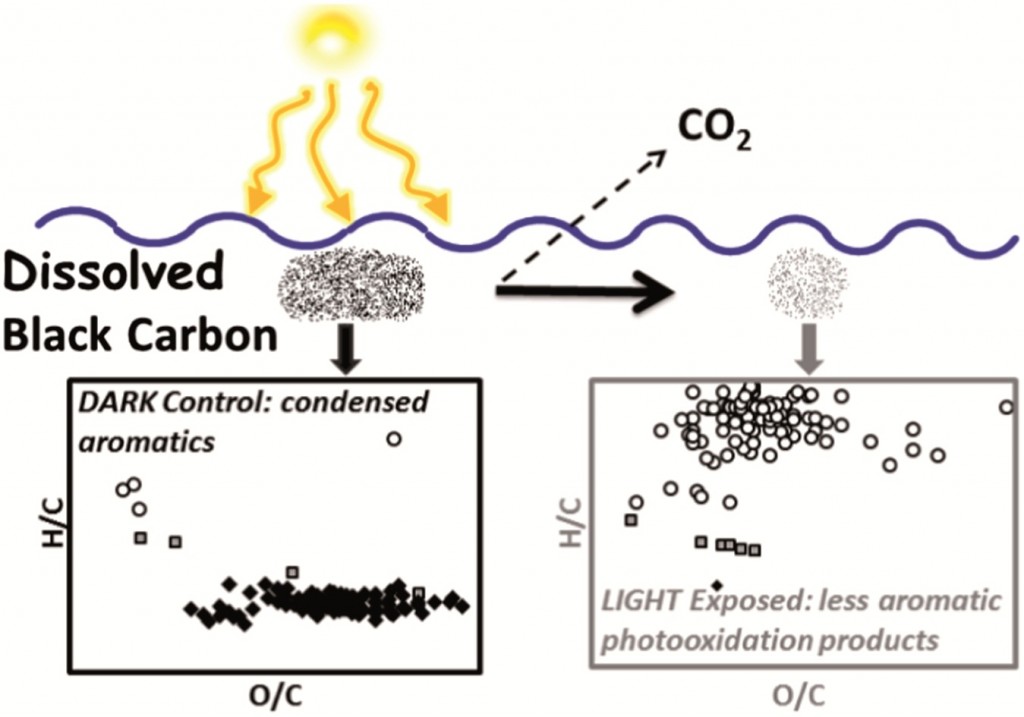
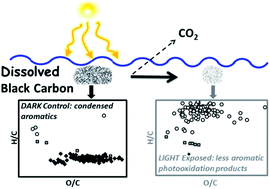
Results indicate BC-d in surface waters was disproportionately more susceptible to photooxidation than BCp, based on O2 consumption and CO2 production during exposure. It was shown that 8-13% of the C in BC-d was photo-mineralized to CO2 while 68-91% was partially oxidized to compounds that remained in solution.
Following exposure to sunlight the majority of identified molecules in the mass spectra were aliphatics (85%) with smaller amounts of condensed aromatics (10%) and aromatics (5%), while in dark controls, most identified compounds were condensed aromatics, suggesting these compounds are being partially oxidised primarily. Indeed, 89% of the condensed aromatics identified in dark controls were absent from the mass spectrum following exposure.
Furthermore, the average O/C of aromatics was shown to decrease for aromatics and aliphatics but increase for condensed aromatics remaining after photooxidation, suggesting condensed aromatics are more susceptible to oxidation.
These results strongly suggest sunlight is a key sink for BC in surface waters and that condensed aromatics are the class of compound primarily involved in these photo-reactions. However, the primary reaction pathway involved partial oxidation to less aromatic photoproducts with unknown susceptibility to further degradation. The authors highlight that there is a need to further investigate the reactivity of BC photoproducts to understand the role of sunlight as a sink for BC in the wider geochemical system.
To downloads the full article for free* click the link below:
Insights into the complete and partial photooxidation of black carbon in surface waters
Collin P. Ward, Rachel L. Sleighter, Patrick G. Hatcher, and Rose M. Cory
DOI: 10.1039/c3em00597f
*Access is free untill 23.04.14 through a registered RSC account – click here to register