Bisphenol A (BPA) is one of the most used industrial chemicals worldwide. Since its introduction in the market in 1959, BPA production has increased steadily and it is forecasted to reach 7.3 million tons by the end of 2023.1 BPA is used for a range of applications: from dental sealants to internal can coatings, electronic equipment and supermarket receipts.2 “In the past years, concerns have been raised over the use of this compound due to its estrogenic effects that can be observed also at low BPA concentration, such as the ones found in the natural environment.2,3 Thus, investigation of natural attenuation processes might help us developing strategies to reduce human exposure to this widespread chemical.
Several literature studies showed that manganese oxides (MnOx)-mediated oxidation represents the main PBA degradation pathway in anoxic conditions.2 This process produces a series of degradation products, including radicals that might couple to dissolved organic matter to form the so-called “bound residues”, unknown high molecular weight products whose long-term environmental risks are still debated.4 A detailed knowledge of the reaction mechanism will therefore allow to predict, and ideally prevent, the formation of degradation products that might be more hazardous than the parent compound.
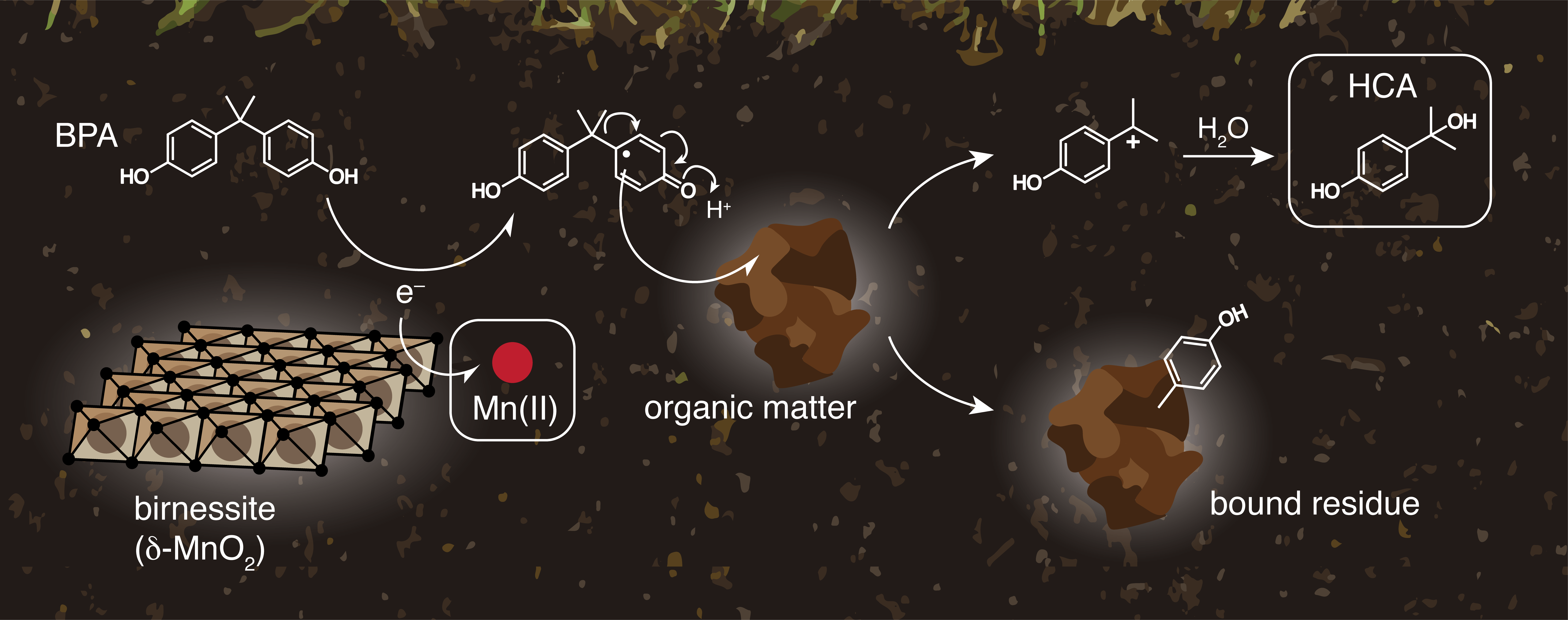
In this context, Balgooyen et al. used stirred flow reactors to investigate the effect of influent concentrations on BPA degradation mechanism via birnessite (δ-MnO2) oxidation. This research question was motivated by the hypothesis that higher influent concentrations might lead to a higher formation of bound residues. The results of this work are directly relevant for engineered water treatment systems that use MnOx-coated sand,5 where contaminant inflow concentrations might change during time.
As a unique feature of this work, the authors used a combined approach based on the detection of both organic and inorganic reaction products. Specifically, they followed the formation of both hydroxycumil alcohol (HCA) and aqueous Mn(II). HCA is the main PBA oxidation product and is considered a proxy for bound residues formation, while Mn(II) is a reaction byproduct released in solution upon reduction of birnessite.
Unexpectedly, the two approaches gave opposite results: HCA yields were constant for the influent concentration range investigated, while Mn(II) yields decreased as the influent concentration increased. In order to explain their results, the authors hypothesized that Mn(II) was not an accurate proxy, as comproportionation and disproportionation reactions occurring at the mineral surface might alter aqueous Mn(II) concentrations. Using an elegant series of sorption and desorption experiments, Balgooyen et al. were able to confirm this hypothesis, leading to the conclusion that BPA oxidation mechanism in stirred-flow reactors is indeed independent from the influent concentration.
In addition to providing a valuable new piece of information for the complex puzzle of BPA cycling in anoxic conditions, the work of Balgooyen et al. teaches us something that has little to do with micropollutants or flow-through reactors: for a throughout study of a chemical mechanism, all reaction partners must be considered – no matter how many different analytical techniques you will have to use.
To download the full article for free*, click the link below:
Sarah Balgooyen, Gabrielle Campagnola, Christina K. Remucal and Matthew Ginder-Vogel
Environ. Sci.: Processes Impacts, 2019, 21, 19
DOI: 10.1039/c8em00451j
Rachele Ossola is a PhD student in the Environmental Chemistry group at ETH Zurich. Her research focuses on photochemistry of dissolved organic matter in the natural environment.
Additional references
(1) The Global Bisphenol A Market, https://www.researchandmarkets.com/reports/4665281/the-global-bisphenol-a-market (accessed May 26, 2019).
(2) Im and Löffler, Environ. Sci. Technol. 2016, 50 (16), 8403–8416.
(3) vom Saal and Hughes, Environ. Health Perspect. 2005, 113 (8), 926–933.
(4) Barraclough et al. Environ. Pollut. 2005, 133 (1), 85–90.
(5) Charbonnet et al., Environ. Sci. Technol. 2018, 52 (18), 10728–10736.
*Article free to access until the 30th June 2019