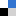Well, not quite. But in recent years researchers have been exploring the potential of using “biochar” to remediate soil contaminated with organic chemicals. Similar to but definitely not the charcoal commonly used during barbecue season, biochar is made by heating biomass such as fruit peels in oxygen-limited conditions. Its physical and chemical characteristics impart an exceptional ability to sorb chemicals, especially organic chemicals, and reduce their bioavailability in soil.
A new study by Xu and co-workers at Peking University and the Chinese Academy of Agricultural Sciences focuses on two widespread organic chemicals: bisphenol A (BPA) and 17α-ethylyneestradiol (EE2). BPA is used for manufacturing polycarbonate plastics and epoxy resins. Thus, it is found in a multitude of commonly used products such as cars, food storage containers, and electronic equipment. EE2 is a synthetic estrogen most commonly used as an ingredient in birth control pills.
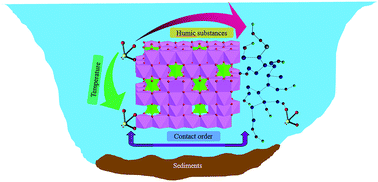
Both of these chemicals have been found to be endocrine disrupters, and can be transported to soils via wastewater irrigation, sludge fertilizers and landfill leachates. As both chemicals are quite hydrophobic, Xu et al. hypothesized that biochar added to soil would significantly sorb BPA and EE2, and as a result would also affect leaching and dissipation of the chemicals.
The researchers tested this hypothesis by adding biochar derived from corn stalks to soil in a series of lab experiments. First, sorption studies involved adding biochar at a level of 4 wt% to soils spiked with 0.01 or 0.1 mg/L of both BPA and EE2, and measuring the amount of the chemicals in both the soil solids and the soil water after equilibrium was established in about 7 days.
They found that the soils containing biochar increased the solid-water distribution coefficients by at least 200% for BPA and EE2 respectively, relative to the soils with no biochar. Next, leaching experiments meant to simulate repeated rainfall events compared biochar-free soils to those with 1, 2 and 4 wt% of biochar, all of which were spiked with BPA and EE2 at levels reflective of environmentally contaminated soils. Biochar-amended soils decreased the amount of leached BPA by 19 to 53% and EE2 by 42 to 77%.
A final set of incubation experiments used soils spiked in a similar manner to those used in the leaching experiments. All soils, including a biochar-free control, were left outdoors at ambient temperatures for three months. Portions of the soils were sampled at 1, 30 and 90 days, and analyzed for their total and bioavailable BPA and EE2 content. The results showed no significant effect on the dissipation of the two chemicals in soil, but large reductions in the bioavailable fractions of BPA and EE2 in soil.
In addition to holding much promise for removing various organic residues from soil, other benefits of biochar in soil include carbon sequestration, reducing greenhouse gas emissions, and improving crop production. The long-term stability of biochar in soil further highlights the multi-faceted potential of biochar as a soil amendment.
To read more about Xu and co-workers’ investigation into biochar’s ability to reduce the mobility of two widespread organic contaminants, download a copy of the full article for free*:
Influence of biochar on sorption, leaching and dissipation of bisphenol A and 17α-ethynylestradiol in soil
N Xu, B Zhang, G Tan, J Li and H Wang
Environ. Sci.: Processes Impacts, 2015, 17, 1722-1730
DOI: 10.1039/C5EM00190K
—————-

About the webwriter
Abha Parajulee is a Ph.D. student at the University of Toronto Scarborough. She is interested in water resources and the behavior of organic contaminants in urban environments.
—————-
* Access is free until 01/12/2016 through a registered RSC account.