The presence of common organic species in the environment could potentially transform metal species from a chemically inert form into more mobile and bioaccessible species, thus influencing the extent and nature of their potential ecological impacts. This collaboration between German and Canadian research institutions demonstrates how this phenomenon can impact the environmental behaviour of palladium, a metal of increasing environmental interest.
The environmental performance of road vehicles has been enhanced in the last 20 years by the use of three-way catalytic converters (TWCCs), which are shown to dramatically reduce emissions of CO2 as well as organic pollutants.
The key active catalyst used in TWCCs is palladium (Pd), either used on its own or in combination with other metals such as platinum (Pt) and rhodium (Rh). Indeed, it is estimated that global consumption of Pd by the catalytic converter industry increased nearly 10-fold between 1993 and 2013. It has been indicated that the increased use of Pd in TWCCs has been accompanied by increasing environmental concentrations of Pd compounds observed in soils, sediments, plants and aquatic ecosystems.
This has therefore led researchers to question the possible post-emission transformation, mobility and bioavailability of Pd in the environment, and the factors influencing this behaviour. This type of research is essential in order to assess and reduce the potential ecological damage caused. It is known that organic constituents commonly present in soil, sediments and aquatic systems can form complexes with metals in the environment, which strongly influences the mobility of metals such as zinc (Zn), iron (Fe) and cadmium (Cd). However, much less is known about the chemical behaviour of Pd-containing compounds in the presence of these naturally-occurring organic substances such as humic acids in the environment , and knowledge regarding the environmental behaviour and mobility of Pd under typical environmental conditions is limited.
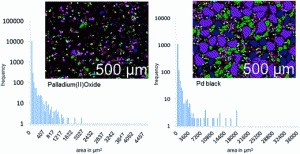
This study by Fathi Zereini and co-workers quantitatively examines the chemical mass transfer, stability and solubility of Pd in the presence of organic complexing agents and the key factors (such as the pH and concentration of organic species) influencing this process, to better understand the potential behaviour of Pd resulting from catalytic converters under typical environmental conditions. The investigation conducted batch experiments using metallic Pd and Pd(II) oxide catalyst particles to investigate the transformation and solubility of these particles in the presence of ethylenediamine tetra acetic acid (EDTA), a common metal chelating agent, which is ubiquitous in the environment.
The results of this experiment demonstrate that, while the EDTA has little impact on the chemical state of Pd oxide, the elemental form of Pd metal used in catalytic converters can be solubilised post-emission under ambient conditions. The pH of Pd-EDTA solutions was shown to modulate Pd solubility and solubility was found to increase with a corresponding increase in the strength of the EDTA concentrations used, in addition to the length of extraction time.
This study therefore indicates that the presence of EDTA can oxidize small amounts of Pd emitted in metallic form into the environment from catalytic converters, thereby contributing to an enhanced mobility and possible bioaccessibility of this metal. These results contrast with previous assumptions that metallic Pd present in soils is chemically inert and immobile.
To access the full article, download a copy for free* by clicking the link below.
The influence of ethylenediamine tetra acetic acid (EDTA) on the transformation and solubility of metallic palladium and palladium(II) oxide in the environment
Fathi Zereini, Clare L. S. Wiseman, My Vang, Peter Albers, Wolfgang Schneider, Roland Schindl and Kerstin Leopold
Environ. Sci.: Processes Impacts, 2015, Advance Article
DOI: 10.1039/C5EM00078e
—————-
Ian Keyte is a Doctoral Researcher at the University of Birmingham. His research focuses on the sources, behavior and fate of polycyclic aromatic hydrocarbons (PAHs) in the atmosphere.
—————-
* Access is free until 27/05/2015 through a registered RSC account.