This month sees the following articles in Energy & Environmental Science that are in the top 25 most accessed from April – June:
Progress in flexible energy storage and conversion systems, with a focus on cable-type lithium-ion batteries
Sang-Young Lee, Keun-Ho Choi, Woo-Sung Choi, Yo Han Kwon, Hye-Ran Jung, Heon-Cheol Shin and Je Young Kim
Energy Environ. Sci., 2013,6, 2414-2423
DOI: 10.1039/C3EE24260A
Graphene-based electrodes for electrochemical energy storage
Chaohe Xu, Binghui Xu, Yi Gu, Zhigang Xiong, Jing Sun and X. S. Zhao
Energy Environ. Sci., 2013,6, 1388-1414
DOI: 10.1039/C3EE23870A
Highly efficient dye-sensitized solar cells: progress and future challenges
Shufang Zhang, Xudong Yang, Youhei Numata and Liyuan Han
Energy Environ. Sci., 2013,6, 1443-1464
DOI: 10.1039/C3EE24453A
3D carbon based nanostructures for advanced supercapacitors
Hao Jiang, Pooi See Lee and Chunzhong Li
Energy Environ. Sci., 2013,6, 41-53
DOI: 10.1039/C2EE23284G
Low-temperature processed meso-superstructured to thin-film perovskite solar cells
James M. Ball, Michael M. Lee, Andrew Hey and Henry J. Snaith
Energy Environ. Sci., 2013,6, 1739-1743
DOI: 10.1039/C3EE40810H
From planar-heterojunction to n–i structure: an efficient strategy to improve short-circuit current and power conversion efficiency of aqueous-solution-processed hybrid solar cells
Zhaolai Chen, Hao Zhang, Xiaohang Du, Xiao Cheng, Xigao Chen, Yingying Jiang and Bai Yang
Energy Environ. Sci., 2013,6, 1597-1603
DOI: 10.1039/C3EE40481A
Development of alternative photocatalysts to TiO2: Challenges and opportunities
María D. Hernández-Alonso, Fernando Fresno, Silvia Suárez and Juan M. Coronado
Energy Environ. Sci., 2009,2, 1231-1257
DOI: 10.1039/B907933E
Light-trapping in dye-sensitized solar cells
Stephen Foster and Sajeev John
Energy Environ. Sci., 2013, Advance Article
DOI: 10.1039/C3EE40185E
Shape-tailored TiO2 nanocrystals with synergic peculiarities as building blocks for highly efficient multi-stack dye solar cells
Luisa De Marco, Michele Manca, Roberto Giannuzzi, Maria R. Belviso, P. Davide Cozzoli and Giuseppe Gigli
Energy Environ. Sci., 2013,6, 1791-1795
DOI: 10.1039/C3EE24345A
Challenges in the development of advanced Li-ion batteries: a review
Vinodkumar Etacheri, Rotem Marom, Ran Elazari, Gregory Salitra and Doron Aurbach
Energy Environ. Sci., 2011,4, 3243-3262
DOI: 10.1039/C1EE01598B
A membrane-free lithium/polysulfide semi-liquid battery for large-scale energy storage
Yuan Yang, Guangyuan Zheng and Yi Cui
Energy Environ. Sci., 2013,6, 1552-1558
DOI: 10.1039/C3EE00072A
Graphene and its derivatives for the development of solar cells, photoelectrochemical, and photocatalytic applications
Da Chen, Hao Zhang, Yang Liu and Jinghong Li
Energy Environ. Sci., 2013,6, 1362-1387
DOI: 10.1039/C3EE23586F
Biomass-derived electrocatalytic composites for hydrogen evolution
Wei-Fu Chen, Shilpa Iyer, Shweta Iyer, Kotaro Sasaki, Chiu-Hui Wang, Yimei Zhu, James T. Muckerman and Etsuko Fujita
Energy Environ. Sci., 2013,6, 1818-1826
DOI: 10.1039/C3EE40596F
Flexible graphene–polyaniline composite paper for high-performance supercapacitor
Huai-Ping Cong, Xiao-Chen Ren, Ping Wang and Shu-Hong Yu
Energy Environ. Sci., 2013,6, 1185-1191
DOI: 10.1039/C2EE24203F
A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density
Fan Zhang, Tengfei Zhang, Xi Yang, Long Zhang, Kai Leng, Yi Huang and Yongsheng Chen
Energy Environ. Sci., 2013,6, 1623-1632
DOI: 10.1039/C3EE40509E
Lithium–oxygen batteries: bridging mechanistic understanding and battery performance
Yi-Chun Lu, Betar M. Gallant, David G. Kwabi, Jonathon R. Harding, Robert R. Mitchell, M. Stanley Whittingham and Yang Shao-Horn
Energy Environ. Sci., 2013,6, 750-768
DOI: 10.1039/C3EE23966G
Photoelectrochemical reduction of nitrates at the illuminated p-GaInP2 photoelectrode
Heli Wang and John A. Turner
Energy Environ. Sci., 2013,6, 1802-1805
DOI: 10.1039/C3EE40745D
Graphene based new energy materials
Yiqing Sun, Qiong Wu and Gaoquan Shi
Energy Environ. Sci., 2011,4, 1113-1132
DOI: 10.1039/C0EE00683A
Stabilizing inorganic photoelectrodes for efficient solar-to-chemical energy conversion
Syed Mubeen, Joun Lee, Nirala Singh, Martin Moskovits and Eric W. McFarland
Environ. Sci., 2013,6, 1633-1639
DOI: 10.1039/C3EE40258D
High-efficiency polymer solar cells with a cost-effective quinoxaline polymer through nanoscale morphology control induced by practical processing additives
Yiho Kim, Hye Rim Yeom, Jin Young Kim and Changduk Yang
Energy Environ. Sci., 2013,6, 1909-1916
DOI: 10.1039/C3EE00110E
High photo-electrochemical activity of thylakoid–carbon nanotube composites for photosynthetic energy conversion
Jessica O. Calkins, Yogeswaran Umasankar, Hugh O’Neill and Ramaraja P. Ramasamy
Energy Environ. Sci., 2013,6, 1891-1900
DOI: 10.1039/C3EE40634B
Review of solutions to global warming, air pollution, and energy security
Mark Z. Jacobson
Energy Environ. Sci., 2009,2, 148-173
DOI: 10.1039/B809990C
High performance hybrid solar cells sensitized by organolead halide perovskites
Bing Cai, Yedi Xing, Zhou Yang, Wen-Hua Zhang and Jieshan Qiu
Energy Environ. Sci., 2013,6, 1480-1485
DOI: 10.1039/C3EE40343B
Carbon nanotubes for lithium ion batteries
Brian J. Landi, Matthew J. Ganter, Cory D. Cress, Roberta A. DiLeo and Ryne P. Raffaelle
Energy Environ. Sci., 2009,2, 638-654
DOI: 10.1039/B904116H
Uniform V2O5 nanosheet-assembled hollow microflowers with excellent lithium storage properties
An Qiang Pan, Hao Bin Wu, Lei Zhang and Xiong Wen (David) Lou
Energy Environ. Sci., 2013,6, 1476-1479
DOI: 10.1039/C3EE40260F
Why not take a look at the articles today and blog your thoughts and comments below
Fancy submitting an article to EES? Then why not submit to us today!











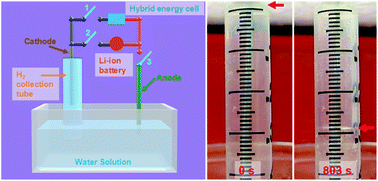


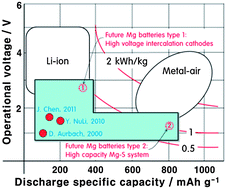 Rechargeable batteries are ubiquitous in portable electronics, and are expected to play an important role in electric vehicles and grid storage in the future. While lithium-ion technology is the current state of the art, concerns remain about the supplies of lithium on Earth. As such, alternative systems such as magnesium-ion batteries are being developed. Magnesium is the fifth most abundant element in the Earth’s crust and its bivalency means that it can in principle store more energy per unit volume than lithium metal.
Rechargeable batteries are ubiquitous in portable electronics, and are expected to play an important role in electric vehicles and grid storage in the future. While lithium-ion technology is the current state of the art, concerns remain about the supplies of lithium on Earth. As such, alternative systems such as magnesium-ion batteries are being developed. Magnesium is the fifth most abundant element in the Earth’s crust and its bivalency means that it can in principle store more energy per unit volume than lithium metal.

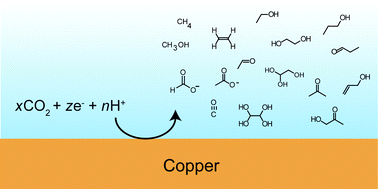 es the work of Jaramillo’s group. The C&EN article focuses on recent developments towards the interesting goal of converting CO2 into useful products using electrochemistry: “Carbon dioxide gets a lot of attention—most of it negative—as a greenhouse gas. But if CO2 could be converted in a cost-effective manner to valuable products, the ubiquitous small molecule so often reviled for its role in climate change might start to lose its bad rap.”
es the work of Jaramillo’s group. The C&EN article focuses on recent developments towards the interesting goal of converting CO2 into useful products using electrochemistry: “Carbon dioxide gets a lot of attention—most of it negative—as a greenhouse gas. But if CO2 could be converted in a cost-effective manner to valuable products, the ubiquitous small molecule so often reviled for its role in climate change might start to lose its bad rap.”