In their recent perspective article in Energy and Environmental Science, Professor Doron Aurbach and co-workers highlight key developments in magnesium-ion battery technology.
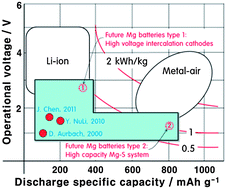
A key requirement in magnesium-ion battery technology is reversible deposition of magnesium on the magnesium metal anode. However, most organic solvents and simple magnesium salts react with magnesium to form a so-called passivation layer on the anode, precluding reversible magnesium deposition and further charge-discharge cycles. Vital progress was made with the discovery of electrolytes that were stable in the presence of the magnesium anode. In particular, reaction of AlCl3-nRn lewis acids with R2Mg lewis bases in ether solvents yielded electrolyte solutions which were stable up to voltages of 2.1 V vs. magnesium metal. The combination of these so-called dicholoro-complex (DCC) electrolytes with Chevrel phase intercalation cathodes (e.g. Mo6S8) and magnesium anodes yielded the first working prototypes for reversible magnesium-ion batteries.
While these first prototypes constituted a great breakthrough, relatively low energy densities and lacking performance at high rates precluded their commercialisation. Ongoing work focuses on finding new electrolytes with wider electrochemical stability windows, as well as new cathode and anode materials. One interesting possibility is to replace the magnesium anode with metal alloys of bismuth and antinomy, sidestepping the problems associated with the reactivity of magnesium and allowing more conventional electrolytes to be used.
Read more in the perspective article in Energy and Environmental Science:
Mg rechargeable batteries: an on-going challenge
Hyun Deog Yoo, Ivgeni Shterenberg, Yosef Gofer, Gregory Gershinsky, Nir Pour and Doron Aurbach
Energy Environ. Sci., 2013, Advance Article
DOI: 10.1039/C3EE40871J
By Alexander Forse










