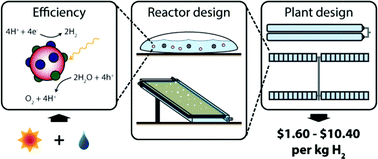
Chemists and chemical engineers from Cal-Tech, Stanford, and the US Department of Energy collaborated to investigate four methods of industrial-scale hydrogen gas generation directly from sunlight, via photocatalysis.
The lowest-tech solution, giant ‘baggies’ containing water and a photocatalytic slurry that co-generate hydrogen and oxygen, is the least expensive option, but in terms of land use is surprisingly as efficient as higher-tech panel-based options. Though this is a technical report, anyone interested in renewable energy and the hydrogen economy on a larger scale would find this interesting.
Hydrogen is the single most promising fuel for the future economy. Although >95% of it is presently made by steam reforming, it can be generated independently from fossil fuels. Thermal reforming of biofuels, renewably powered electrolysis, and photolytic generation together represent the possibilities for renewable H2 generation, and this paper investigates the viability of the last, modelling the scale-up of present technologies for H2 generation to ten tonnes per day. The US Department of Energy (DoE) guidelines set $2-4/kg H2 as a reasonable threshold for viability; based on an ideal location for gathering solar energy, the authors found that two of the four designs viably meet this goal.
Photocatalysis couples the hydrogen evolution reaction (HER) to the oxygen evolutio
lving the proposed overall efficiency. The first design predominates, and is proposed to produce H2 at $1.60/kg.n reaction (ORR). Two designs are based on slurries of photocatalytically active particles in water-filled HDPE bags. The single-bed system evolves H2 and O2 simultaneously, posing an explosive risk and requiring separation, but is simple and cost-effective. The dual-bed system evolves the two gases separately, but requires both the redox reaction of an efficiently redox-cycling ‘mediator’ (e.g. Fe2+/Fe3+) to be coupled to the OER and HER reactions and porous bridges between half-cells to transport the mediator between the cells, significantly complicating the design and halving the efficiency.
The other two designs are based on panels composed of two photocatalytically active layers between a transparent anode exposed to the sun where the HER occurs and a metal cathode where the OER occurs. The third design involves flat panels. The fourth design uses cylindrical parabolas for a 10:1 light concentration to increase efficiency. In both designs, the two reactions are inherently separated, and although panels are more efficient for the area collecting sunlight, panel spacing makes their footprint larger. Of these, the parabolic design predominates, producing at $3.20/kg.
Thus, both the giant baggie and concentrator panel options are economically viable – the baggies are cheaper, but the panels have fewer unknowns in terms of their safety, lifetime, and mass production. Compare these efficiencies with those of electrolyzer apparatuses paired to solar electrical generation, a further study the authors recommend, and we’ll have a good picture of the future of renewable fuel generation.
Read the article in EES:
Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry
Blaise A. Pinaud, Jesse D. Benck, Linsey C. Seitz, Arnold J. Forman, Zhebo Chen, Todd G. Deutsch, Brian D. James, Kevin N. Baum, George N. Baum, Shane Ardo, Heli Wang, Eric Miller and Thomas F. Jaramillo
DOI: 10.1039/C3EE40831K, Analysis