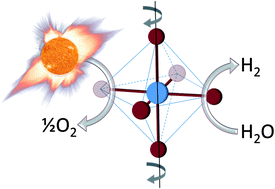
The researchers identified an improved catalyst that splits water (or carbon dioxide) after heating by concentrating solar: using mirrors or lenses to shine lots of sunlight on one spot to get it really hot. At high temperatures (traditionally above 1500°C), oxygen is liberated from the catalyst. The catalyst is then cooled (traditionally to 800°C) and exposed to water vapor (or carbon dioxide gas). Starved for oxygen, the catalyst steals it from the water (or carbon dioxide) to leave hydrogen (or carbon monoxide). These products may either be directly used as fuel or for synthesis of more traditional fuels such as gasoline.
The improved catalyst is from a class of materials called “perovskite oxides” and contains a mix of strontium, lanthanum, manganese, aluminum and oxygen. Compared to traditional catalysts that use cerium or iron, this improved catalyst performs over a smaller range of temperatures: 1350°C for oxygen liberation and 1000°C for hydrogen (or carbon monoxide) production. This decrease in temperature range is important to ensuring the life of the catalyst, which (cycled every 5 minutes for 8 hours per day, 300 days per year over 10 years) must be sturdy enough to withstand 300-thousand cycles. It is anticipated that further exploration into this class of catalysts will yield further improvements.
Read the article in EES today:
Sr- and Mn-doped LaAlO3−δ for solar thermochemical H2 and CO production
Anthony H. McDaniel, Elizabeth C. Miller, Darwin Arifin, Andrea Ambrosini, Eric N. Coker, Ryan O’Hayre, William C. Chueh and Jianhua Tong
DOI: 10.1039/C3EE41372A











