Posted on behalf of Stuart Bartlett, web writer for Dalton Transactions
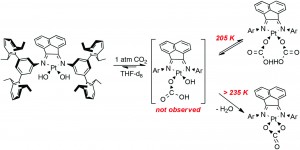
The capture of inert gases, such as CO2, is a fundamental process across biology and transition metal chemistry. There are many advantages to CO2 fixation; one important example is to reduce greenhouse gas emissions from waste streams. The Piers group in Calgary have found that platinum(II) complexes with sterically imposing diimine ligands can undergo CO2 insertion reversibly into a Pt-OH bond to give new carbonate ligands.
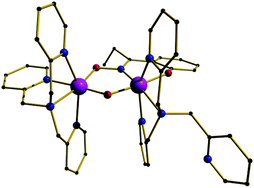
These complexes are easily synthesised from a platinum precursor, trans-[Pt(SMe2)2Cl(R)] {R = Me, Ph} , in the presence of a diimine ligand followed by chloride abstraction using Ag2O/H2O to give [Pt(NN)(OH)(R)] {NN = Diimine-(3,5-bis-(2,6-diisopropylphenyl)benzene)}. Also, by using a dichloride precursor, the dihydroxo species can also be obtained. This dihydroxo species was found to react immediately with 1 atm CO2 to yield [(NN)Pt(CO3-η2)] nearly quantitatively. At a lower temperature of 205 K, NMR studies showed the presence of a [(NN)Pt(CO3H)2] complex, which reverted back to [(NN)Pt(CO3-η2)] when warmed up. This process is thought to occur via de-insertion of CO2, to give the initial Pt-OH bond, followed by H2O elimination back to [(NN)Pt(CO3-η2)].
Analysis of the mixed hydroxo-alkyl species, [Pt(NN)(OH)(R)], showed ~50% insertion of CO2 into the Pt-OH bond at room temperature, increasing to 100% at 260 K. Using NMR analysis, this was observed to be a fully reversible process upon heating back to room temperature, giving the initial [Pt(NN)(OH)(R)] complex. Alkane elimination (R-H) was not observed in any circumstances, whereas CO2 elimination as seen as the more favoured route. Also at the lower temperatures, where the CO2 is retained, the alkane elimination barrier is thought to be too high.
This research displays how important transition metal chemistry can be in reactions of chemically inert species, such as CO2, at normal temperatures. This reversible capture points to many important areas, such as biological reactions of enzymes, possible pathways to develop new synthons in organic chemistry and industrial applications for reversible CO2 capture.
Find out more from the article:
Reversible insertion of carbon dioxide into Pt(II)–hydroxo bonds
Tracy L. Lohr, Warren E. Piers and Masood Parvez
Dalton Trans., 2013, 42, 14742-14748
DOI: 10.1039/C3DT51701B
 Stuart Bartlett is currently doing a 1 year postdoc position with David Cole-Hamilton at the University of St Andrews, focusing on the conversion of renewable oils towards fine chemical production using metathesis. He obtained his PhD from the University of Southampton investigating the mechanism of ethene oligomerisation catalysis using NMR and X-ray Absortion Spectroscopy.
Stuart Bartlett is currently doing a 1 year postdoc position with David Cole-Hamilton at the University of St Andrews, focusing on the conversion of renewable oils towards fine chemical production using metathesis. He obtained his PhD from the University of Southampton investigating the mechanism of ethene oligomerisation catalysis using NMR and X-ray Absortion Spectroscopy.