Posted on behalf of Liana Allen, web writer for Dalton Transactions
Protein purification is an essential step in the study and characterisation of naturally occurring molecules and the understanding of their functions in different biological processes. Before an in-depth study can be achieved, the protein of interest needs to be separated not only from the non-protein constituents of the tissue or cell culture, but also from any other proteins present. The latter is usually the most difficult aspect of the separation process.
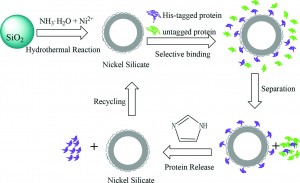
Protein tagging is a common way of separating target proteins from other biological materials. In an example of this tagging method, target proteins are deliberately expressed with histidine (His) residues, which have a high affinity for binding to metal ions. This technique is popular due to its easy adaptation to any protein tagging system, however it also has limitations including long operation times and the necessity for pretreatment of the organic matter. Recently, this separation procedure has been improved by immobilising the metal ions on solid supports such as silica nano-spheres, though low surface area and low surface metal ion density have thus far limited practical application of this.
In this paper, the authors report an efficient synthesis of nickel functionalised silica nano-spheres and demonstrate their superior performance in the affinity purification of His-tagged proteins. By first chemically affixing nickel ions to the surface of silica nano-spheres, then removing the silica cores, hollow spheres are created, with greater surface area and higher Ni2+ surface density than the current nano-particle materials used for protein separation. The authours demonstrate that His-tagged TRX proteins could be separated directly from crude E. coli cell material using their hollow, Ni2+ functionalised nano-spheres. Moreover, after washing and sonicating, the nano-spheres could be reused up to five times while maintaining the same high efficiency.
To read more, see:
Preparation of hollow nickel silicate nanospheres for separation of His-tagged proteins
Yonghui Wu, Guanxiao Chang, Yanbao Zhao and Yu Zhang
Dalton Transactions, 2013, Advance Article, DOI:10.1039/c3dt52084f
 Dr. C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds.
Dr. C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds.