This month sees the following articles in Dalton Transactions that are in the top ten most accessed:-
The end of iodide? Cobalt complex redox shuttles in DSSCs
Thomas W. Hamann
Dalton Trans., 2012, 41, 3111-3115 DOI: 10.1039/C2DT12362B
Enhanced cytotoxicity of silver complexes bearing bidentate N-heterocyclic carbene ligands
Diana C. F. Monteiro , Roger M. Phillips , Benjamin D. Crossley , Jake Fielden and Charlotte E. Willans
Dalton Trans., 2012, 41, 3720-3725 DOI: 10.1039/C2DT12399A
A series of isostructural mesoporous metal–organic frameworks obtained by ion-exchange induced single-crystal to single-crystal transformation
Qingxia Yao , Junliang Sun , Kuo Li , Jie Su , Maxim V. Peskov and Xiaodong Zou
Dalton Trans., 2012, 41, 3953-3955 DOI: 10.1039/C2DT12088G
Highly-connected, porous coordination polymers based on [M4(µ3-OH)2] (M = CoII and NiII) clusters: different networks, adsorption and magnetic properties
Qing Chen , Wei Xue , Jian-Bin Lin , Rui-Biao Lin , Ming-Hua Zeng and Xiao-Ming Chen
Dalton Trans., 2012, 41, 4199-4206 DOI: 10.1039/C2DT12022D
Functional porphyrinic metal–organic frameworks: crystal engineering and applications
Chao Zou and Chuan-De Wu
Dalton Trans., 2012, 41, 3879-3888 DOI: 10.1039/C2DT11989G
Synthesis and characterization of a new porphyrin–polyoxometalate hybrid material and investigation of its catalytic activity
Mehdi Araghi , Valiollah Mirkhani , Majid Moghadam , Shahram Tangestaninejad and Iraj Mohammdpoor-Baltork
Dalton Trans., 2012, 41, 3087-3094 DOI: 10.1039/C2DT11865C
A copper thiolate centre for electron transfer: mononuclear vs. dinuclear complexes
Marcello Gennari , Jacques Pécaut , Marie-Noëlle Collomb and Carole Duboc
Dalton Trans., 2012, 41, 3130-3133 DOI: 10.1039/C2DT12355J
Sequential self-assembly in metal–organic frameworks
Brandon J. Burnett and Wonyoung Choe
Dalton Trans., 2012, 41, 3889-3894 DOI: 10.1039/C2DT12103D
Synthesis of porous aromatic framework with tuning porosity via ionothermal reaction
Wei Wang , Hao Ren , Fuxing Sun , Kun Cai , Heping Ma , Jianshi Du , Huijun Zhao and Guangshan Zhu
Dalton Trans., 2012, 41, 3933-3936 DOI: 10.1039/C2DT11996J
A fluorescence “turn-on” chemodosimeter for Cu2+ in aqueous solution based on the ion promoted oxidation
Junbo Li , Yang Zeng , Qihui Hu , Xianglin Yu , Jia Guo and Zhiquan Pan
Dalton Trans., 2012, 41, 3623-3626 DOI: 10.1039/C2DT12497A
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Dalton Transactions? Then why not submit to us today or alternatively email us your suggestions.
Comments Off on Top ten most accessed articles in February
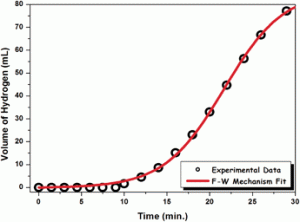 Amine borane complexes are a promising solution for storing hydrogen, particularly as we move towards a hydrogen economy. Whilst focusing on developing the dehydrogenation of such complexes (namely dimethylamine-borane), scientists from Turkey have shown that you need to think small to think big by preparing a nanoparticle catalyst for the reaction.
Amine borane complexes are a promising solution for storing hydrogen, particularly as we move towards a hydrogen economy. Whilst focusing on developing the dehydrogenation of such complexes (namely dimethylamine-borane), scientists from Turkey have shown that you need to think small to think big by preparing a nanoparticle catalyst for the reaction.











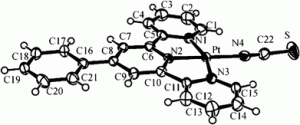
![solvent accessible voids in [Pt{4'-(Ph)trpy}(NCS)]SbF6 solvent accessible voids in [Pt{4'-(Ph)trpy}(NCS)]SbF6](https://blogs.rsc.org/dt/files/2012/03/Pt-terpyridine-complex-solvent-300x145.gif)