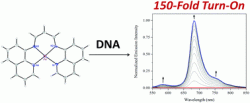
Stephen Lippard and colleagues at Massachusetts Institute of Technology have synthesised and characterised a platinum(II) complex bearing the tetradentate, β-diketiminate ligand ([Pt(BDIQQ)]Cl). Cytotoxic studies showed [Pt(BDIQQ)]Cl to have similar potency to cisplatin on cervical cancer (HeLa) cells, whereas on human lung carcinoma (A549) cells [Pt(BDIQQ)]Cl was four times more potent than cisplatin. The complex elicits a strong photoluminescent turn-on response upon DNA binding and it is hoped this will be useful for further cellular imaging studies.
Read the article now to find out more…
Photoluminescent DNA binding and cytotoxic activity of a platinum(II) complex bearing a tetradentate β-diketiminate ligand
Jennifer M. Hope, Justin J. Wilson and Stephen J. Lippard
Dalton Trans., 2013
DOI: 10.1039/C2DT32462H, Communication
Other Dalton Transactions articles by this author include:
A C2-symmetric, basic Fe(III) carboxylate complex derived from a novel triptycene-based chelating carboxylate ligand
Yang Li, Justin J. Wilson, Loi H. Do, Ulf-Peter Apfel and Stephen J. Lippard
Dalton Trans., 2012,41, 9272-9275
DOI: 10.1039/C2DT31260C, Communication
Non-traditional platinum compounds for improved accumulation, oral bioavailability, and tumor targeting
Katherine S. Lovejoy and Stephen J. Lippard
Dalton Trans., 2009, 10651-10659
DOI: 10.1039/B913896J, Perspective
From themed issue Metal anticancer compounds