Scientists in Germany and Japan have shown that cerium(IV) predominantly forms a dinuclear complex in aqueous solution. Until now, scientists had thought that tetravalent cerium was a monomeric species in aqueous solution. The finding could have important implications for scientists studying water oxidation.
Atsushi Ikeda-Ohno from the Helmholtz-Zentrum Dresden Rossendorf, Dresden, and colleagues used a combination of extended X-ray absorption fine structure and density functional theory calculations to study the cerium(IV) cation. ‘In an aqueous solution, the primary form of metal cations is generally a simple mononuclear hydrate complex or a mononuclear oxo-cation,’ says Ikeda-Ohno, ‘but cerium(IV) forms a unique oxo- or/and hydroxo-bridging dinuclear complex.’
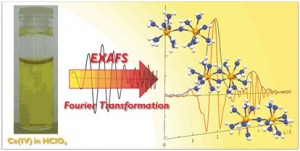
A combined X-ray absorption spectroscopy and density functional calculation study has revealed that a single oxo-bridging dinuclear complex is the dominant form of soluble Ce(IV) species in an aqueous perchloric acid solution.
‘We have always assumed that any reaction between a catalyst and a cerium salt is a one electron transfer reaction,’ says Curtis Berlinguette from the University of Calgary, Canada, who studies the mechanistic behaviour of homogeneous water oxidation catalysts using cerium salts as the terminal oxidant. ‘But if cerium(IV) does exist as a dimer and has an oxo bridge, then it really does question these assumptions and, more importantly, the nature of the oxygen-oxygen bond formation step.’
Berlinguette explains that the current assumption is that you make a catalyst with the metal oxo unit and that reacts with water. But, if the cerium salt that exists in solution contains an oxo, then you could potentially have a combination between the cerium(IV) oxo and the metal oxo, producing oxygen. Therefore, what might appear to be a water oxidation catalyst is in fact just forming dioxygen through reaction with the cerium salt.
‘It would be really interesting if Ikeda-Ohno’s team could extend this study to the acidic conditions and concentrations that are more widely used by the homogeneous catalytic water oxidation community. Then we will have much more insight into what species we are actually studying in solution,’ says Berlinguette.
Ikeda-Ohno says that developing time-resolved systems for X-ray techniques to probe the reactions of the cerium(IV) is the team’s next challenge.
Written by Rachel Cooper
Dinuclear complexes of tetravalent cerium in an aqueous perchloric acid solution
Atsushi Ikeda-Ohno, Satoru Tsushima, Christoph Hennig, Tsuyoshi Yaita and Gert Bernhard
Dalton Trans., 2012, Advance Article
DOI: 10.1039/C2DT12406H











![solvent accessible voids in [Pt{4'-(Ph)trpy}(NCS)]SbF6 solvent accessible voids in [Pt{4'-(Ph)trpy}(NCS)]SbF6](https://blogs.rsc.org/dt/files/2012/03/Pt-terpyridine-complex-solvent-300x145.gif)