When metal nanoparticles are used as catalysts, they can have a tendency to aggregate. Especially when the metal nanoparticles (NPs) are not functionalized as there isn’t anything to oppose the Van der Waals forces. Aggregation or coalescence of the NPs can lead to a decrease in catalytic activity. Two strategies have been developed for stabilisation — addition of a ligand post NP synthesis or inclusion of a coordinating ligand into the cation of an ionic liquid (IL).
In a previous Dalton Transactions article, (Dalton Trans., 2011, 40, 4660–4668) the stability of ruthenium nanoparticles (RuNPs) was improved by the combination of ILs and stabilising ligands to produce a recyclable catalytic system for the hydrogenation of toluene. In this new article, Agilio Padua and colleagues have taken this one step further and investigated the effect of various ligands on the catalytic activity of RuNPs in an IL.
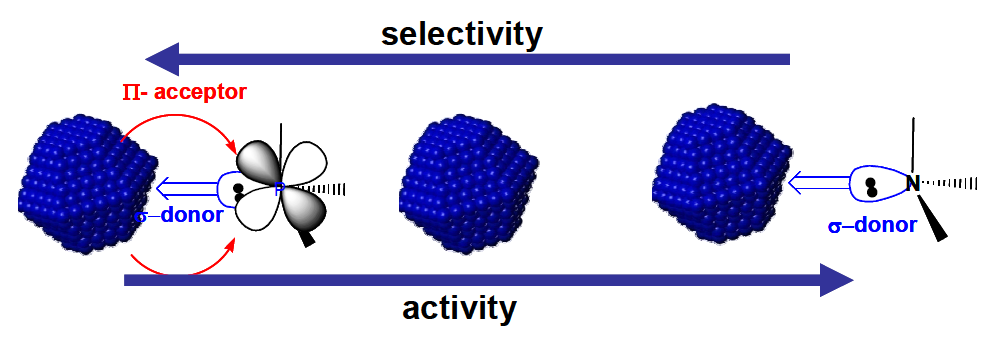
RuNPs were prepared in an IL, and in the presence and absence of a series of different ligands. Padua and colleagues discover that the activity of RuNPs with respect to the catalytic hydrogenation of cyclohexadiene, styrene and limonene increased with σ-donor ligands (water and octylamine) but decreased with π-acceptor ligands (carbon monoxide, phenylphosphine and diphenylphosphine). This paper demonstrates how RuNPs as catalysts are remarkably similar to homogenous catalysts in that their activity and selectivity can be controlled by σ-donor and π-acceptor ligands.
Download the full paper to find out more…
Ligand effect on the catalytic activity of ruthenium nanoparticles in ionic liquids
Santini Catherine, Paul S Campbell, Gorka Salas, Karine Philippot, Agilio A.H. Padua and Margarida Costa Gomes
Dalton Trans., 2012