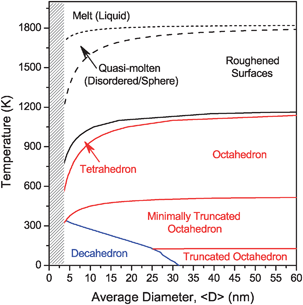
There’s always room for improvement and with this at the forefront of their minds, Giuseppe Bellussi and colleagues from Eni, Italy have focused on “converting the bottom of the barrel” – that is, turning high molecualr weight oil fractions into lighter compounds in the more useful boiling point range.
There are several different technologies that address this (see the Catalysis Science & Technology article for more information) but Bellussi et al. have concentrated their efforts on upgrading the slurry bed technology – a process that so far has seen limited industrial development.
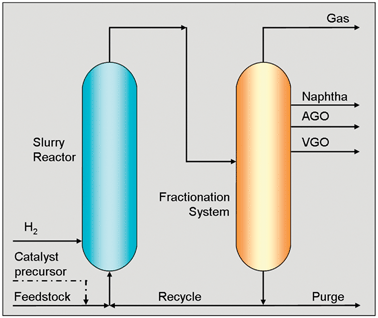
The slurry technology that Eni put their cracking catalyst/Mo2S catalyst to use in
The key to their success in fully converting the heavy and extra heavy oils into the middle distillates was using a dual catalyst – a catalytic system which comprises both a conventional cracking catalyst and nano dispersed molybdenum disulfide, the latter of which is responsible for protecting the cracking catalyst from coke and metal deposition.
For more details, download the article now…
The role of MoS2 nano-slabs in the protection of the heterogeneous cracking catalyst for the total conversion of heavy oils to good quality distillates
Giuseppe Bellussi, Giacomo Rispoli, Daniele Molinari, Alberto Landoni, Paolo Pollesel, Nicoletta Panariti, Roberto Millini and Erica Montanari