|
Sara Coles is a guest web-writer for Catalysis Science & Technology. She currently works for Johnson Matthey in Royston, UK. |
Porous carbon nanostructures can be excellent catalyst supports, especially for nanoparticles of noble metals such as palladium.
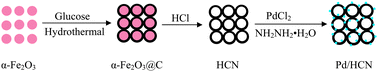
A paper co-authored by Maiyong Zhu and colleagues, in China, describes the use of pre-synthesised α-Fe2O3 nanoparticles as templates to form hollow carbon ‘nanonets’ on which palladium nanostructures are deposited by an in situ precipitation-reduction procedure. The advantage of the hollow nanonet structure is that a higher catalyst loading can be achieved, potentially leading to greater activity towards the target reaction.
The researchers tested their supported palladium catalysts for the Suzuki and Heck coupling reactions, with good yields although the conversions of substituted substrates tended to be lower than unsubstituted ones. The reactions could also be carried out in water – good news for ‘green’ chemistry.
Compared to supports based on solid carbon spheres, the nanonet supported catalysts had slightly higher palladium loadings and considerably better catalytic performance.
The group have also confirmed through experimental methods that the reactions are indeed catalysed by the supported palladium and not by any leached palladium in solution. The catalysts could be recycled, though there was some loss of activity. Analysis showed that after the Heck reaction, in particular, there was significant aggregation of palladium nanoparticles, thought to be due to temperature effects, as well as deformation of the nanonet carbon structure.
Read more detail about this work in Catalysis Science & Technology:
Hematite nanoparticle-templated hollow carbon nanonets supported palladium nanoparticles: preparation and application as efficient recyclable catalysts
Maiyong Zhu, Ying Wang, Chengjiao Wang, Wei Li and Guowang Diao
Catal. Sci. Technol., 2013, 3, 952-961, DOI: 10.1039/C2CY20562A