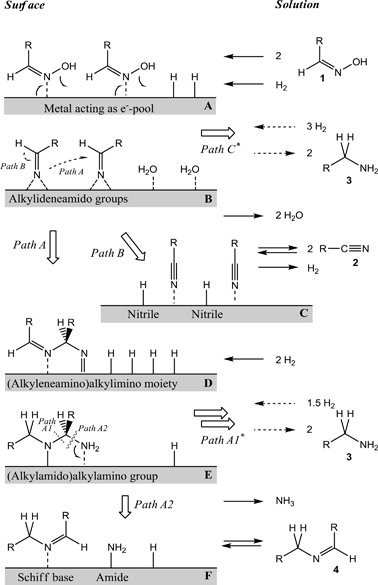
Thomas Müller and colleagues have investigated the mechanism by which oximes are hydrogenated, usually giving a mixture of both primary and secondary amine products. Since primary amine functionality is desireable for many fine chemicals, it would be ideal to find a way to selectively generate primary amines in preference to their secondary relatives.
They disovered that the reaction proceeds via a pool of Schiff base and nitrile intermediates which can be directed towards primary or secondary amines depending on the choice of catalyst – first-row transition metal catalysts such as nickel encourage primary amine formation, whilst noble metal catalysts (Pd, Rh) encourage secondary amine formation.
To read about the reaction mechanism in detail, download the Catalysis Science & Technology article now…
Controlling Selectivity in the Reaction Network of Aldoxime Hydrogenation to Primary Amines
Ewa Gebauer-Henke, Walter Leitner, Angelina Prokofieva, Henning Vogt and Thomas Ernst Mueller