PCCP Advisory Board members are occasionally invited to select a few personal ‘highlights’ from recent issues of the journal. I received such a call in autumn 2012, and readily agreed to try and pick out ten particularly noteworthy papers in the general areas of photochemistry and spectroscopy.
PCCP Advisory Board members are occasionally invited to select a few personal ‘highlights’ from recent issues of the journal. I received such a call in autumn 2012, and readily agreed to try and pick out ten particularly noteworthy papers in the general areas of photochemistry and spectroscopy.
I had long penciled in the Christmas break as the first realistic opportunity for this task. I have been involved with PCCP in one way or another since its launch in 1999, so I should know better than most just how successful a journal it has now become. But it very soon became apparent that I would need to constrain my selection more tightly than I had originally imagined. PCCP publishes a lot of papers (1843 in 2012, many of which impinge on areas of photochemistry and spectroscopy, and many of which certainly merit very careful study. So, my first self-imposed (and completely arbitrary) constraint was that only papers carrying a (print) publication date of 2012 would be eligible for inclusion in my ‘Editor’s Choice’ list. This still left the challenge of somehow picking out less than one in every hundred papers!
The next obvious challenge was that Vol. 14 contains several themed issues, two of which – on Ultrafast chemical dynamics and on Hydrogen bonding in electronically excited states – could easily provide more than ten personal highlights. In both cases, the guest editors did an exemplary job securing many new, high quality articles. I thoroughly recommend both of these themed issues to all who share my scientific interests, and claim writer’s prerogative by counting each as just one of my ten! I applied one more filter, and excluded from consideration the several excellent papers published in PCCP by Bristol colleagues during 2012.
The 2012 collection includes many notable articles offering new insights into photo-induced processes (e.g. photofragmentations, studies of intramolecular energy flow, photoionization, etc) and into the dynamics of bimolecular encounters – some with unprecedented definition of the initial (reactant) and final (product) quantum states. From the many excellent gas phase studies, I eventually selected:
M.P. Grubb, M.L. Warter and S.W. North, ‘Stereodynamics of multistate roaming’, which reported clear correlations between the velocity vector v and the angular momentum vector j of the NO fragments from NO3 photolysis, implying that the final intra-molecular abstraction in this roaming ‘reaction’ is constrained to occur at planar geometries;
C. Logé and U. Boesl, ‘Laser mass spectrometry with circularly polarized light: two-photon circular dichroism’ demonstrating two-photon circular dichroism of isolated gas phase molecules (cyclopentadienone) by multiphoton ionization measurements;
and the perspective article C.D. Lin and Junliang Xu, ‘Imaging ultrafast dynamics of molecules with laser-induced electron diffraction’, as a new route to imaging the ultrafast dynamics of small molecules with sub-Å spatial and a few-fsec temporal resolution.
My personal interests are now evolving to include photo-induced processes in solution, with particular emphasis on studies that reveal dynamical information about such processes. Obviously, there already exists a very rich and extensive literature pertaining to this field – which I am working hard to absorb – but the 2012 issues of PCCP provide many further articles that I have now come to regard almost as essential reading. The following five complete my ‘Editor’s Choice’ list:
B. Abel, U. Buck, A. L. Sobolewski and W. Domcke, ‘On the nature and signatures of the solvated electron in water’ – a perspective article that reviews recent measurements of the binding energy of hydrated electrons by liquid-jet photoelectron spectroscopy, and considers their implications for our understanding of electron solvation in aqueous environments;
R.R. Frontiera, C. Fang, J. Dasgupta and R.A. Mathies, ‘Probing structural evolution along multidimensional reaction coordinates with femtosecond stimulated Raman spectroscopy’. Another excellent perspective article, in which the authors demonstrate how the exquisite time and frequency resolution afforded by fsec stimulated Raman spectroscopy has allowed them to follow structural evolutions in an isomerization, and in selected electron and proton transfer reactions;
O. Braem, T.J. Penfold, A. Cannizzo and M. Chergui, ‘A femtosecond fluorescence study of vibrational relaxation and cooling dynamics of UV dyes’– that reports a fsec broad-band fluorescence up-conversion study that explores details of the (contrasting) vibrational relaxation dynamics of two UV chromophores;
K.M. Lange, A. Kothe and E.F. Aziz, ‘Chemistry in solution: recent techniques and applications using soft X-ray spectroscopy’. Another perspective article, this one highlighting some of the new opportunities for studying the structure and dynamics of chemical and biochemical systems in solution offered by time-resolved soft X-ray absorption and emission spectroscopies;
F. Zamponi, J. Stingl, M. Woerner and T. Elsaesser, ‘Ultrafast soft-mode driven charge relocation in an ionic crystal’ – a short paper that illustrates some of the exciting structural and dynamical insights offered by the latest generation fsec X-ray laser pulses in powder diffraction measurements of an ionic solid (potassium dihydrogen phosphate in this case).
Read Professor Ashfold’s Editor’s choice selection for free for a limited period:
Stereodynamics of multistate roaming
Michael P. Grubb, Michelle L. Warter and Simon W. North
DOI: 10.1039/C2CP40235A
Laser mass spectrometry with circularly polarized light: two-photon circular dichroism
Christoph Logé and Ulrich Boesl
DOI: 10.1039/C2CP41405H
Imaging ultrafast dynamics of molecules with laser-induced electron diffraction
C. D. Lin and Junliang Xu
DOI: 10.1039/C2CP41606A
On the nature and signatures of the solvated electron in water
B. Abel, U. Buck, A. L. Sobolewski and W. Domcke
DOI: 10.1039/C1CP21803D
Probing structural evolution along multidimensional reaction coordinates with femtosecond stimulated Raman spectroscopy
Renee R. Frontiera, Chong Fang, Jyotishman Dasgupta and Richard A. Mathies
DOI: 10.1039/C1CP22767J
Chemistry in solution: recent techniques and applications using soft X-ray spectroscopy
Kathrin M. Lange, Alexander Kothe and Emad F. Aziz
DOI: 10.1039/C2CP24028A
A femtosecond fluorescence study of vibrational relaxation and cooling dynamics of UV dyes
Olivier Braem, Thomas J. Penfold, Andrea Cannizzo and Majed Chergui
DOI: 10.1039/C2CP23167K
Ultrafast soft-mode driven charge relocation in an ionic crystal
F. Zamponi, J. Stingl, M. Woerner and T. Elsaesser
DOI: 10.1039/C2CP24072F
And don’t forget to check out his themed issue highlights:
Ultrafast chemical dynamics
Guest Editors: Klaas Wynne (University of Glasgow, UK) and Neil T. Hunt (University of Strathclyde, UK).
Hydrogen bonding in electronically excited states
Guest Editors: Guang-Jiu Zhao and Ke-Li Han (Dalian Institute of Chemical Physics, China)
Comments Off on Professor Mike Ashfold picks out his Editor’s Choice on photochemistry and spectroscopy


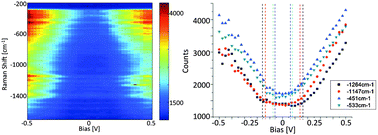










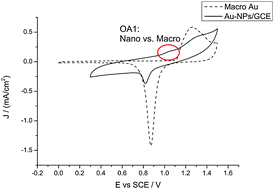
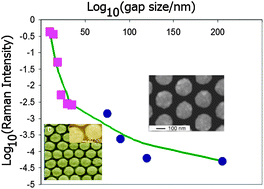
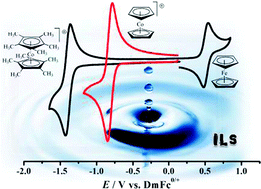 Four classical metallocene redox couples are investigated in this paper. Ferrocene/ferrocenium is the reference redox couple of choice, but has been shown to be sensitive so specific solvation and ion pairing. This is also the case for ionic liquids, just much more extreme. In the four studied ionic liquids the variation is a full 100 mV. A similar large variation is found for cobaltocenium/cobaltocene. This variation presents a major issue if either of these redox couples are used as an internal reference in electrochemical experiments.
Four classical metallocene redox couples are investigated in this paper. Ferrocene/ferrocenium is the reference redox couple of choice, but has been shown to be sensitive so specific solvation and ion pairing. This is also the case for ionic liquids, just much more extreme. In the four studied ionic liquids the variation is a full 100 mV. A similar large variation is found for cobaltocenium/cobaltocene. This variation presents a major issue if either of these redox couples are used as an internal reference in electrochemical experiments.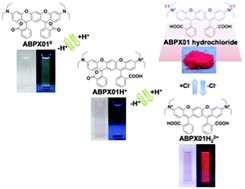 The new, extended or double, rhodamine dye shows interesting properties, as a consequence of the bulk of the molecular structure and the elongation and extension of the conjugated system. A small red-shift of the absorption maximum of 50 nm occurs, and the new molecular structure allows the xanthenium fluorophore to be strongly emissive in the solid state.
The new, extended or double, rhodamine dye shows interesting properties, as a consequence of the bulk of the molecular structure and the elongation and extension of the conjugated system. A small red-shift of the absorption maximum of 50 nm occurs, and the new molecular structure allows the xanthenium fluorophore to be strongly emissive in the solid state.