In their recent PCCP communication, Richard Compton et al. explore gold electrocatalytic activity and report very interesting differences between the macro-and nano-scales.
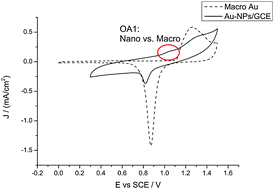 The researchers from Oxford University, UK, applied the procedure of consecutive electro-oxidation and reduction cycling in sulphuric acid medium to electrodeposited nanoparticles. Whereas this method is commonly used as a cleaning and calibration procedure for gold macro-electrodes, the method was found to have a negative effect on the surface of gold nanoparticles.
The researchers from Oxford University, UK, applied the procedure of consecutive electro-oxidation and reduction cycling in sulphuric acid medium to electrodeposited nanoparticles. Whereas this method is commonly used as a cleaning and calibration procedure for gold macro-electrodes, the method was found to have a negative effect on the surface of gold nanoparticles.
It has previously been thought that this surface cleaning method can be effectively applied to gold nanoparticles on the assumption that their behaviour is the same as the bulk behaviour. Compton et al. correctly question this assumption and suggest that changes in the surface morphology and/or composition of the nanoparticles during the cycling treatment may cause the damaging effects on the gold nanoparticle-modified electrode.
Read more detail in this article today:
Surface oxidation of gold nanoparticles supported on a glassy carbon electrode in sulphuric acid medium: contrasts with the behaviour of ‘macro’ gold
Ying Wang, Eduardo Laborda, Alison Crossley and Richard G. Compton
DOI: 10.1039/C3CP44615H










