Ionic liquids are the new black; a nonsense statement, but very true. Recyclable ionic liquids are the new solvents of choice as they allow for extensive recycling, novel processes and treatments and higher durability of devices. Ionic liquids are also just new, with unexplored physical and chemical properties. In a recent PCCP paper Torriero and co-workers target the issue of referencing electrochemical measurements in ionic liquids.
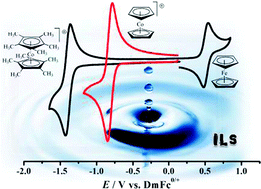
The per-methylated derivatives sandwich complexes, derived from 1,2,3,4,5-pentamethyl-cyclopentadienyl or Cp* (Cp-star), vary, but vary less. The relative change, measured as the difference in reduction potential of between Cp*2Fe and Cp*2Co+, is only a few millivolts. The result presented in this paper shows that the Cp* sandwich complexes may show promise as the new standard in electrochemical experiments in ionic liquids.
The limitation of the suggested internal references are discussed in full in:
Assessment of permethylated transition-metal sandwich complexes as internal reference redox systems in ionic liquids
Angel A. J. Torriero, Jaka Sunarso, Maria Forsyth and Cristina Pozo-Gonzalo
Phys. Chem. Chem. Phys., 2013, 15, 2547-2553
DOI: 10.1039/C2CP43177G
by Dr Thomas Just Sørensen










