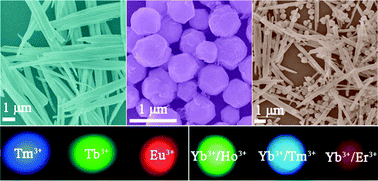
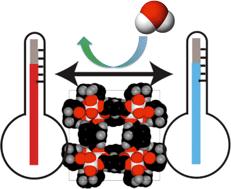
Nano-sized crystals (or nanocrystals) have better solubilities and dissolution characteristics than larger crystals do. Preparation of nanocrystals of drug molecules is therefore of interest to the pharmaceutical industry, particularly in cases where poor solubility is an issue. However, preparation of crystals of the desired size and crucially, the correct polymorph, is not straightforward. Problems include the long times required and the unwanted formation of amorphous material. One promising method of nanocrystal preparation is to grow crystals in pores where the size of the pores limits the size of the crystals formed.
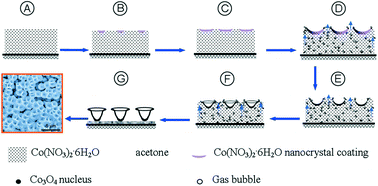
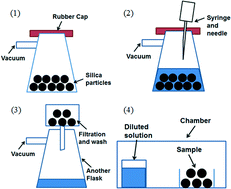
A recent paper in CrystEngComm by Myerson and co-workers reports the growth of three nanocrystals of pharmaceutical ingredients (APIs) – ibuprofen, fenofibrate and griseofulvin – using silica, where the pores of the silica structure provide so-called rigid confinement. The formation process is simple, involving loading of the API into the silica, washing to remove any API adhering to the surface (rather than inside the pores, see diagram below), crystallisation and drying. These steps can be varied to optimise the outcomes.
The nanocrystals exhibit enhanced solubilities and improved stabilities, due to the protection offered from e.g. moisture by the pores. The pores also limit possible reorganisations which would result in undesired crystal forms. The authors also highlight that the samples can be directly formulated into capsules without any additional steps, decreasing the formulation time significantly.
Read the full paper for more information:
Formation of organic molecular nanocrystals under rigid confinement with analysis by solid state NMR
X. Yang, T. C. Ong, V. K. Michaelis, S. Heng, J. Huang, R. G. Griffin and A. S. Myerson
CrystEngComm, 2014, DOI: 10.1039/C4CE01087F, Paper
___________________________________________________________________________________________________
 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.











 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.