Calcium phosphate (CaP) based materials have long been popular choices for a range of medical applications including bone replacement and drug delivery. However, recent studies indicate a need for a closer look at how cells react to small, degraded CaP particles that find their way into the cell’s interior. In a recent study, a research team from the University of Birmingham demonstrated that when CaP particles with a diameter larger than 1.5μm penetrated the interior of the cell but were not sequestered by the cell’s lysosomes, a series of events eventually leading to cell death could be observed.
Within the past few years, an increased focus on the end results of CaP degradation have shown that the eventual cytotoxicity of these materials is heavily dependent on the total volume of internalized material. A study by Motskin et al. in 2009 illustrated that many of these degraded particles are localized to the lysosomes, cellular structures whose acidic environments are responsible for the degradation of waste products which, in the case of CaP, results in the generation of calcium ions. It was suggested that the generation of a surplus of these ions could interfere with the cell’s signalling pathways, potentially triggering apoptosis.
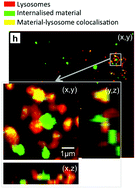
In a study published recently in Biomaterials Science, Williams et al. quantified the volume and size distribution of CaP material within cells by chemically grafting a fluorescent probe to the surface of silicon-substituted hydroxyapatite (SiHA), and then exposing a mouse osteoblast precursor cell line to the labelled particles. Following exposure, the group observed changes in cell behaviour that were indicative of the onset of cell death including alterations in cell morphology, an increase in the number of lysosomes, and cellular detachment from the underlying substrate. As the cells transitioned from the early stages of cell death (morphology changes) to later stages (detachment from the substrate), there was a marked increase in the amount of SiHA within the cytoplasm but outside of the lysosomes. Moreover, the onset of cell death was correlated with SiHA aggregates within the cytoplasm that were 1.5μm in diameter or larger.
The group hypothesized that this may have been due to a destabilization of the lysosome membrane which prevented undissolved CaP from remaining within the lysosomes or, alternatively, the destabilization of endosomes which were responsible for delivering particles to the lysosomes. Regardless, this study proves a need for additional research into the size-dependent effects of CaP particles on cell health and suggests a new design concern for CaP based medical materials.
Check out the full article here:
Quantification of volume and size distribution of internalised calcium phosphate particles and their influence on cell fate
by Richard L. Williams, Isaac Vizcaíno-Castón, and Liam M. Grover
Ellen Tworkoski is a Web Writer for Biomaterials Science and a graduate student in the department of Biomedical Engineering at Northwestern University.