Cancer is the second leading cause of death worldwide, and continues to be a challenging disease to treat therapeutically. One strategy to treat cancer patients is to surgically remove the primary tumor and attempt to prevent the spread of cancer cells to other organs in the body. Unfortunately, surgical resection of tumors may be difficult in sensitive organs (e.g. the brain), and tumors may need to be treated directly with chemotherapy drugs to inhibit growth.
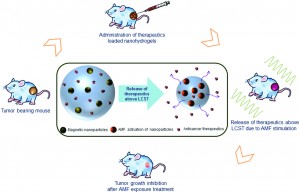
Recently, there has been an increased interest in utilizing biomaterials to deliver chemotherapy drugs directly to the primary tumor. Current research at the Indian Institute of Technology explores the use of nanohydrogels supplemented with iron oxide magnetic nanoparticles as a vehicle to deliver chemotherapy drugs to the primary tumor. Interestingly, these thermo-responsive nanohydrogels could release their anticancer drug cargo when heated with a magnetic field. Although this study did not use chemotherapy drugs, the magnetic hydrogels were used to heat the tumor locally to reduce tumor size – upon stimulation with a magnetic field. This study focused on characterizing the material properties of the magnetic nanohydrogels, in addition to analyzing the effects of the nanohydrogel on inhibiting tumor growth.
The magnetic nanohydrogel is composed of iron oxide nanoparticles, chitosan, and poly-N-isopropylacrylamide (NIPAAm). The chitosan-poly-(NIPAAm) nanohydrogels are hydrophilic below the lower critical solution temperature (LCST) of 42oC. When stimulated with the appropriate magnetic field, the magnetic nanoparticles heat the hydrogel above the LCST. When the temperature is increased above the LCST, the polymers in the hydrogel change from “expanded coils” to “compact globules,” and cause the release of the aqueous contents of the hydrogel. The hydrophilic to hydrophobic transition is the main mechanism by which the anticancer drug cargo could be released from the hydrogel.
The biocompatibility and efficacy of the hydrogel in reducing tumor growth in vivo were analyzed. Using a mouse model of fibrosarcoma, the magnetic nanohydrogels were delivered via injection to the primary tumor site, and then stimulated with a magnetic field. Various biocompatibility studies were conducted, showing that serum protein concentrations and blood cell counts were not affected. The biodistribution of the magnetic hydrogel was also quantified, with minimal accumulation of the nanohydrogel in various organs (14 days post-delivery). Additionally, the magnetic nanohydrogel decelerated the growth of the primary tumor. After magnetic stimulation, the magnetic particles in the nanohydrogel successfully heated the tumor locally with minimal damage to the surrounding tissues. Despite not containing chemotherapy drugs, the magnetic nanohydrogels were still efficient in inhibiting the primary tumor growth.
This study describes the thermo-responsive properties of magnetic nanohydrogels, and demonstrates the ability to reduce tumor size using nanohydrogels heated with a magnetic field. Future studies are needed to show efficacy in delivering chemotherapy drugs using the magnetic nanohydrogels as a delivery vehicle. Still, the magnetic nanohydrogels are a promising platform for a minimally invasive method of decreasing tumor growth in vivo.
Biocompatibility, biodistribution and efficacy of magnetic nanohydrogels in inhibiting growth of tumors in experimental mice models
Manish K. Jaiswal, Manashjit Gogoi, Haladhar Dev Sarma, Rinti Banerjee and D. Bahadur
Biomater. Sci., 2014, Advance Article DOI: 10.1039/C3BM60225G
Brian Aguado is currently a Ph.D. Candidate and NSF Fellow in the Biomedical Engineering department at Northwestern University. He holds a B.S. degree in Biomechanical Engineering from Stanford University and a M.S. degree in Biomedical Engineering from Northwestern University. When he’s not in the lab, Brian enjoys traveling, cooking, swimming, and spending time with family and friends. Read more about Brian’s research publications here.
To keep up-to-date with all the latest research, sign-up to our RSS feed or Table of contents